
There are some strange uses for a spent tea bag after it’s made your cuppa, but work published in Scientific Reports really takes the biscuit. A group of researchers in Korea have managed to demonstrate an enhanced carbon anode structure from waste tea leaves that could be a path to cheap, high-capacity lithium ion batteries.
Lithium ion batteries power everything from mobile phones to electric cars. They normally consist of a lithium cathode and a graphite anode in an organic solvent. The graphite anode is cheap, stable and has a suitable electrochemical potential, but it poses a fundamental limit to battery capacity in terms of how many lithium ions it can absorb. If lithium ion batteries are to meet the growing demands for an energy storage solution that can power efficient vehicles and allow renewable energy to displace fossil fuels, then a better anode material must be found.
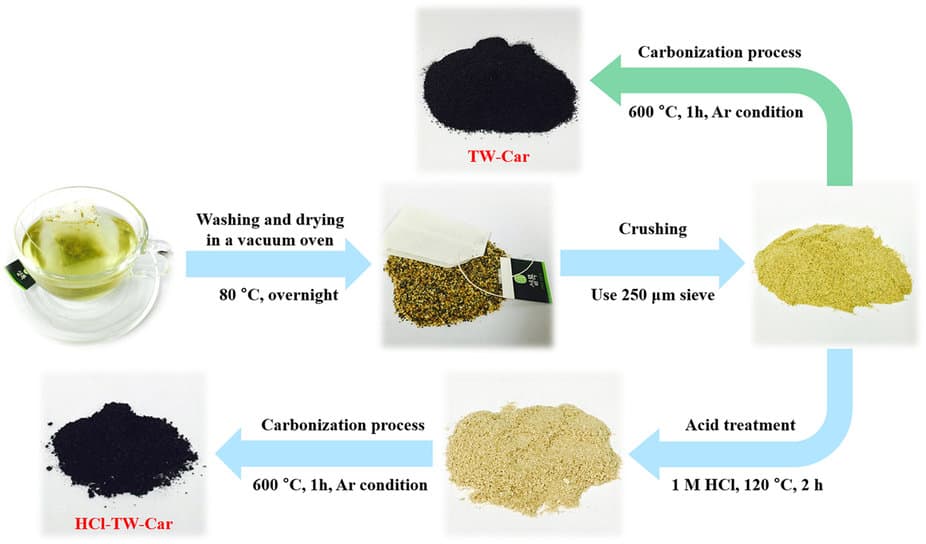
Some of this work towards better anode materials focusses on developing a porous carbon anode that can maintain the chemical advantages of graphite while benefiting from the higher surface area of the porous nanostructure to improve on its capacity limitation. These methods are held back from mass production by the need for high-quality carbon and complex manufacturing processes. However a recent study led by Dong-Wan Kim at Korea University demonstrates how an appropriate porous carbon structure can be made cheaply through a simple series of steps applied to waste tea leaves.
The added acid advantage
Kim and colleagues at Korea University and Korea Institute of Science and Technology, compare two methods. In both, the tea is washed, dried, crushed and carbonized, but one also includes an acid treatment step with hydrochloric acid. They found that the acid treatment step improved the material in a number of ways.
The acidification process seemed to remove unwanted impurities. Traces of several metals amounting to 1% by weight in the untreated sample were not found at all in the acidified sample. What’s more the structure of the pores was different. The unacidified sample had low porosity with relatively large pore size (average 5.69 nm). The acidified carbon had a hierarchically porous structure with a mixture of larger and smaller pores, higher porosity and an average pore size of 2.48 nm. This had the effect of increasing the surface area by almost a factor of 100.

To compare the electrical performance of the two prospective anode materials, Changhoon Choi – the Korea University researcher who performed and analysed the experiments – used them to make lithium ion coin cells. The acid-treated material showed capacities of 479 mAhg-1, higher than the upper limit of 372 mAhg-1 for graphite anodes and the capacity of 270 mAhg-1 for the sample with no acid treatment. After 200 charging and discharging cycles both types of cell showed stable efficiency of charge transfer above 98.5 %, indicating that the reversibility of this electrode is very good and highlighting what good use can be made of an abundant resource – waste tea leaves.



