Scientists in the US are the first to combine infrared and X-ray lasers to study the electronic properties of matter. The technique involves firing infrared light at a diamond sample that is also illuminated by X-rays. Some of the light is absorbed by the diamond’s valence electrons and its energy is then transferred to some of the X-rays scattering from the sample. This allows the team to differentiate between X-rays that have interacted with valence electrons and X-rays that have scattered from the sample’s core electrons – something that has never been done before.
X-ray diffraction involves bouncing X-rays off the electron clouds that surround a material’s constituent nuclei and studying the interference patterns that are created. While it gives a wealth of information about the structure and composition of materials, the technique reveals little about the sample’s chemically active valence electrons. This is because the majority of electrons involved in the scattering are “core” electrons, which do not take part in chemical processes.
More than 40 years ago, Isaac Freund and Barry Levine at Bell Labs proposed a way of getting round this problem. They pointed out that if the sample is exposed to laser light, the valence electrons will respond by oscillating at the laser frequency. Some of the oscillation energy is then transferred to the X-rays as they scatter from the valence electrons in a process called “wave mixing”. As a result, X-rays scattered from the valence electrons will emerge at a slightly higher energy that is equal to the sum of the incoming X-ray and laser energies.
High intensity needed
The effect is small, however, and seeing it requires an extremely intense X-ray beam – something that is only now available at the Linac Coherent Light Source (LCLS) at the SLAC National Accelerator Laboratory in California, where this latest work was done by Thornton Glover and colleagues. The team studied diamond because the material’s structural and electronic properties are already well known. While not a laser in the conventional sense, the LCLS is called a free-electron laser (FEL) because it produces laser-like X-ray pulses that are highly coherent.
To study valence electrons, the team fires simultaneous 8 keV X-ray pulses and infrared pulses at the sample. Most of the X-ray beam undergoes normal diffraction and leaves the diamond sample at a specific angle. However, some of the X-ray beam absorbs energy from the valence electrons and is slightly boosted in energy. These X-rays leave the sample at a slightly different angle and are directed through an aperture that blocks out the much more intense diffracted beam.
By measuring the intensity of the energy-boosted X-rays as a function of the scattering angle, the team was able to work out the density of valence electrons along a specific direction of the diamond lattice. The result agreed with what we already know about carbon, showing that the technique works as expected.
Computer simulations
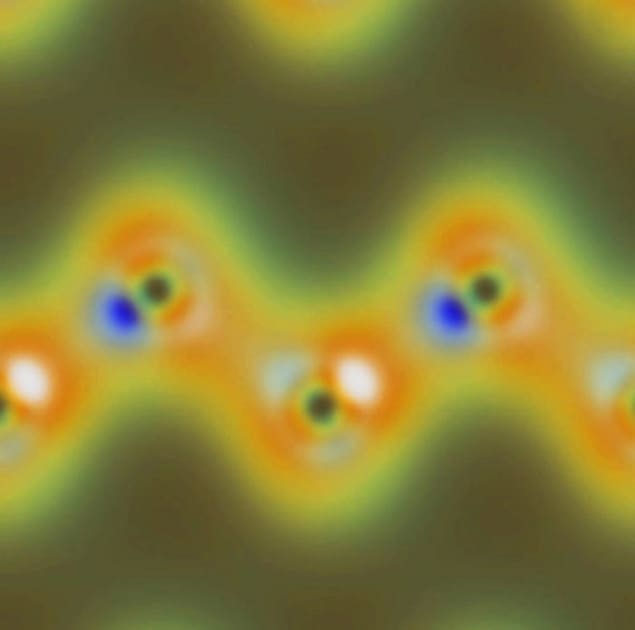
In order to get a complete 3D image of the valence-electron density, the measurement would need to be repeated at a number of different orientations of the diamond crystal – measurements that the team has not yet reported. However, based on their preliminary results, Glover and colleagues have done computer simulations that suggest such measurements should provide maps of valence bonds within the diamond crystal (see image).
Now that it has been shown that wave-mixing measurements can be made using the LCLS, Glover believes that the technique could be used to study a range of materials. “The easiest kinds of diffraction experiments are with crystals, and there’s lots to learn,” he says. “For example, light can be used to alter the magnetic order in advanced materials, yet it’s often unclear just what the light does, on the microscopic scale, to initiate these changes.”
Shedding light on photosynthesis
Looking beyond crystalline materials, Glover also believes that the technique could shed light on photosynthesis, in which photon energy is converted to chemical energy and then transferred in processes that occur on picosecond timescales. “Quantum entanglement plays an important role [in photosynthesis], as an excited electron simultaneously samples many spatially separated sites, probing to find the most efficient energy-transfer pathway,” explains Glover. “It would be great if we could use X-ray and optical wave mixing to make real-space images of this process as it’s happening, to learn more about the quantum aspects of the energy transfer.”
However, Glover points out that such a measurement would require X-ray lasers with much higher repetition rates than are currently available. “FELs of the future will combine high peak brightness with a high repetition rate, and this combination will open up new opportunities for examining the interactions of light and matter on the atomic scale.”
The research is described in Nature.



