Improved integrated implants and less invasive surgical techniques may be available thanks to work recently published in PNAS. Researchers in Paris have shed light on how the movement of water from hydrogel devices to tissues is key to achieving and controlling adhesion. The results provide insights into the role of interfacial transport in bioadhesion and hold promise for better strategies to fix devices to internal organs.
A huge number of implants and surgical devices are made from hydrogels, 3D polymeric networks capable of absorbing large amounts of water. These biocompatible materials not only make up implants, but are also used as protective layers, substrates for biosensors and platforms for drug delivery. For all of these applications the hydrogel must be fixed to soft and wet internal organs. This can be difficult. Bioadhesive surfaces are much preferred because mechanical fixing can damage both the device and the tissue. However, the extremely wet interface means that adhesion can also be tricky as surrounding biological fluid and constant vascular flow inhibits binding.
A sticking point
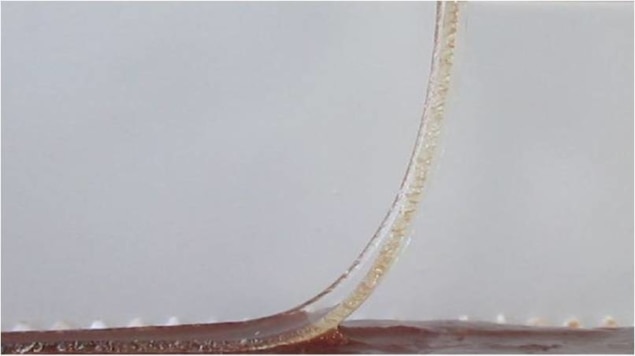
Laurent Corté and his team characterized the adhesion between animal livers and model hydrogel membranes by a peeling test to help understand the role of water transport in attaching devices. They found that the contact time, the amount of water already contained in the hydrogel and the hydration state of the tissue are all critical for adhesion. The group were able to identify two regimes. When the hydrogel is only in contact for a short time, is already extensively swollen with water or the tissue is drier, adhesion is low and liquid wets the interface (lubricated regime). However, after longer contact times, with a less extensively swollen hydrogel and hydrated tissues, solid binding occurs (adhesive regime).
Corté and his team determined that adhesion depends on whether the hydrogel can soak up all of the surrounding biological fluids. For example, in the liver this is blood and bile. The group were then able to devise a simple model for the transition between the lubricated and adhesive regimes.
If the hydrogel is not able to absorb all of the free water, this leaves a liquid film on the interface, preventing bonding between the two surfaces. However, when the hydrogel can absorb all of the free water, the interface is completely drained. This allows the crucial short-range interactions between the tissue and the hydrogel network to form. At this point, the hydrogel can dehydrate the cells themselves at the surface of the tissue. The group liken the tissue as it starts to dehydrate to intrinsically sticky pressure-sensitive adhesives.
Corté and his team showed that this even holds true in vivo, where biological fluids are constantly being replenished, so that draining the interfacial fluid by the hydrogel is more difficult.
Hydrogel material flexes its muscles
Improving adhesion
From these new insights the team were able to show improved bioadhesion using superabsorbent membranes capable of absorbing a lot of water and swelling quickly.
Furthermore, by identifying the huge improvement in adhesion by local dehydration of tissues, this work opens up the possibility of tailoring the fixing of hydrogel-based devices and implants. The researchers suggest that these insights could also be combined with existing binding approaches, opening routes for functional strategies. This could help to fine-tune strength and durability for specific applications and tissues. The team also suggest that these findings and predictions can be applied to many other internal organs.