Collective cell migration – in which cells associated in tight or loose groups move together – plays a central role in embryonic development, wound repair and cancer invasion. As the physical mechanism behind this process remains puzzling, with different aspects to be elucidated, a team of researchers from the University of Arizona has now shown how individual cell biophysics actually dictates the collective behaviour (Biophys. J. 113 1613).
For wound healing, sheet migration is observed. Here, the sheet moves forward as cells at the front edge, the leaders, guide the rest of the cells towards advancing and efficient repairing. As collective behaviour is indeed the result of individual actions, Charles Wolgemuth and his group studied how single epithelial cells influence collective dynamics in wound healing.
Substrate impacts single cell motility
Upon culturing epithelial (MDCK type I and II) cells on different substrates that alter cell-surface adherence (fibronectin, FN, and poly-L-lysine, PL), the authors analysed changes in the cells’ dynamics and morphology. While the two cell types are closely related, the results revealed that highly dynamic MDCK I cells suffered a decrease in area when on PL, suggesting a decrease in adhesion. MDCK II cells, on the other hand, were unaffected by the substrate.
One would expect that cells that move faster when isolated (MDCK I moved faster on FN than on PL, while MDCK II were slower than MDCK I regardless of substrate) would move faster together as well. But when Wolgemuth and his team investigated wound-healing dynamics using scratch assays (creating a “scratch” in the cell monolayer), they found that there was no correspondence between the speed of isolated cells and their collective dynamics.
Are leader cells or jamming the cause?
The dynamics of wound healing is often attributed to leader cells, which stabilize the border and determine the directed migration of the follower cells. The authors showed that the frequency of leader cells did not influence how fast the wound healed, except for MDCK II cells cultured on PL, indicating a correlation between the number of leader cells and coordinated movements in this case.
The researchers next investigated whether collective cell migration is related to a jamming transition, a process in which cells that are packed together tightly can shift to a migratory state as a unit. By taking into consideration a model in which cells display a jamming transition from a solid-like to a fluid-like state (Phys. Rev. X 6 021011), the authors found that MDCK I cells, either on FN or PL, do not represent a jammed system. As such, the physics of jamming cannot be applied for understanding the collective motions of these cells.
Contractile stress imposes collective migration
A model for wound healing described by Lee and Wolgemuth (Biophys J. 111 256) implies that contractile stress, produced by intracellular contraction, is involved in collective migration and can lead to fluid-like flows in an epithelial monolayer. By using this model, the authors simulated a confluent layer of epithelial cells and found that “the contractile stress is the more dominant factor in controlling collective migration speed than the isolated cell velocity”.
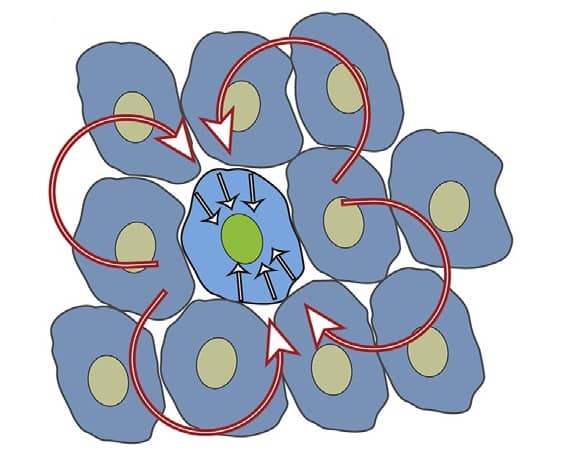
Furthermore, by analysing collective motion in confluent layers of MDCK cells cultured on different substrates, Wolgemuth and his team found out that MDCK I cells on PL had different dynamics in comparison with the other experimental conditions. Such cells undergo highly coordinated motion with larger velocities within the monolayer, enabling them to migrate further than the other cells.
Additionally, the authors used drugs that weaken or strengthen the cytoskeleton to see the effect of contractile stress on MDCK I cells on PL. The results indicated a decreased or increased intralayer speed, respectively, in accordance with the model that showed a “monotonically increasing dependence of intralayer speed on contractile stress” for these cells.
This study reveals important information regarding collective migration dynamics, emphasizing that cell speed is not the driving force that lies behind wound healing but that intracellular contraction is the dominant factor in collective movements. Moreover, the results raise into question the use of wound-healing assays as a measure of cell motility, since substrates can interfere with collective dynamics and should not be used to study single-cell processes.