Wires just three atoms wide that change from being insulators to electrical conductors – and then back again – when struck by a laser pulse have been created by researchers in Germany. The team has shown that the phase transitions can occur as fast as quantum mechanics allows, something that was not previously thought possible. The technique could prove useful in the study of a wide range of systems including how proteins rearrange themselves.
Phase transitions are ubiquitous in all forms of matter. If energy is added to or removed from a system, the most stable state may change: ice melts to liquid water when heated, for example. Subtler phase transitions can also occur within one state: solid iron can exist in several different crystal structures, for example. The speed at which such phase transitions can occur normally depends on how fast energy can enter the crystal lattice, stimulating random motion of the atoms, for example by scattering of electrons. Previous research by Michael Horn-von Hoegen of the University of Duisburg-Essen in Germany and others has shown that this can occur within 2–5 ps in bulk materials, but takes longer at surfaces because of weak coupling between bulk and surface vibrational modes.
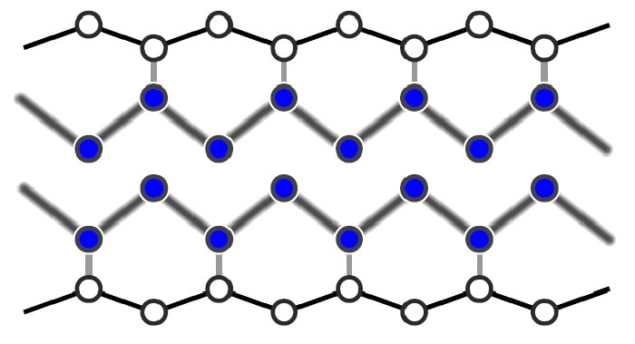
In the new research, however, Horn-von Hoegen and colleagues have shown that surface phase transitions can occur much faster than this. The team studied indium atoms adsorbed onto silicon surfaces. At high temperatures the atoms self-assemble into metallic wires just three atoms wide, whereas at temperatures below 125 K, the wires break up and the surface becomes an insulator.
Diffraction pattern
The researchers first cooled their indium-on-silicon sample to 30 K and measured the electron diffraction pattern of the insulating surface. They then hit the surface with near-infrared laser pulses, causing the surface to warm up. After a variable time delay, they used electron pulses to see how the diffraction pattern had changed. For delays longer than 350 fs, the diffraction pattern of the insulating state was replaced by that of the metallic wires.
“We can answer the question ‘How fast do the atoms move and how fast are they accelerated during this displace excitation mechanism?'” explains Horn-von Hoegen. “It’s something like a trillion times faster than the acceleration you can obtain with a racing car.” The speed increased with the power of the laser pulse up to a certain point, above which it remained constant. “That is then the so-called quantum limit,” says Horn-von Hoegen: “The system cannot react any faster.”
Computational modelling by theoreticians at the University of Paderborn suggests that indium electrons are photoexcited to higher energy levels in the heating process. “We populate electronic states that weaken some bonds or strengthen non-existing bonds,” explains Horn-von Hoegen. This drives the atomic motion during the phase transition. Furthermore, as the atoms move, the electronic band structure of the electron system changes. Incredibly, all of this happens while the atoms are still cold: “Only on a timescale a factor of six to 10 slower does the lattice heat up,” he explains.
Metastable metal
The theoreticians calculated the overall potential energy of the system for different electronic excitations. From this they extracted the conversion time from the insulating to the metallic state, which matched the observed value. After the transition, the cold system lacks the activation energy needed to return to the insulating state, so remains in the metastable metallic state for about 10 ns, which team member Tim Frigge describes as “eternity” for such an atomic system. Despite this delay, the system can be thought of as the fastest electronic switch ever observed, However, Horn-von Hoegen stresses the paper is fundamental research: “I think the idea that these atomic wires will at some point be used as interconnects in ultrafast optical switches should not be taken too seriously,” he admits.
Claus Ropers of the University of Göttingen in Germany is impressed: “There’s not that much structural-dynamics research yet conducted at surfaces because the technology is still being developed,” he says. “These authors have made significant contributions in the past, and in the combination of theoretical and experimental work, this is probably the paper where they’ve put together the most comprehensive understanding of a particular transition.”
Bradley Siwick of McGill University in Canada agrees: “It’s probably the most exciting and the best work yet done on ultrafast dynamics in a surface system,” he says. “We [the scientific community] can watch atoms, follow their motion on the fastest timescales open to them, and see what effect those rearrangements have on the electronic properties of materials. This is a remarkable step forward. This is just the tip of the iceberg: experiments like this could allow you to follow the structure of a protein in a crystal as a function of time and determine how that protein performs its function at the atomic level.”
The research is described in Nature.



