Just as electrons can be excited inside an atom, neutrons and protons can be excited from their ground states into higher-energy states inside the nucleus. If these states have long half-lifes then they are called nuclear isomers. In general such long-lived nuclear states are also associated with other unusual properties. In addition to their fundamental interest, however, nuclear isomers can also be thought of as energy stores: a single nucleus can hold up to several mega-electron-volts. This means that one gram of material could store several giga-joules of energy.
If it were possible to release the enormous energy stored in an isomer in a controlled way, we would have prodigious energy densities at our fingertips. It now appears that Carl Collins of the University of Texas at Dallas and co-workers from Romania, Russia, the Ukraine and the US have managed to achieve this feat. They bombarded an isomer of hafnium-178 with X-rays, and observed an increase in the number of high-energy gamma-rays released by the isomer (Phys. Rev. Lett. 1999 82 695). A deeper understanding of these nuclear isomers and their decay modes could pave the way to novel applications including gamma-ray lasers and, possibly, space travel.
The hafnium-178 isomer studied by Collins and co-workers has an energy of 2.4 MeV and a half-life of 31 years. This combination of high excitation energy and long half-life is unique in nuclei. The isomer can be produced in several different ways, for example by bombarding naturally occurring ytterbium-176 with alpha particles. Having obtained the hafnium-178 sample, Collins irradiated it with a 15 mA dental X-ray machine. The X-rays, which had a peak flux at 40 keV, increased the decay rate of the isomer by 4 ± 2%. The 2.4 MeV of energy is carried away by several lower-energy gamma-rays.
Taken in isolation, one would not want to bet a lot of money on being able to exploit these gamma-rays. However, Collins and co-workers show some rather convincing gamma-ray spectra, and it should be relatively easy to generate a more powerful X-ray beam in future experiments. Collins is careful not to overstate the general significance of the measurement, but his team includes rocket scientists who would like to capitalize on the huge energy density of nuclear isomers. Such isomers may have the potential to provide new ways of propelling spacecraft on interplanetary voyages. Isomers could also form the basis of a gamma-ray laser. The energy inversion that is essential to all laser operation occurs naturally in nuclear isomers, although the route to an actual gamma-ray laser has yet to be mapped out.
What has been shown for the first time is that a large energy gain can be achieved. On the basis that a 40 ± 20 keV X-ray photon can release 2.4 MeV of gamma-ray energy, Collins quotes an energy gain of 60. But could the isomer be persuaded to release its energy by even lower-energy photons? Could the 2.4 MeV isomer be de-excited with visible photons, which have energies in the eV range? Is an energy gain of one million achievable?
This might sound like speculation but it may be possible to investigate the interactions of photons with energies in the eV range with nuclei in experiments. At first sight this seems a hopeless task because the wavelength of visible light is about 108 times larger than an atomic nucleus. However, although any direct interaction would be negligible, the light can couple strongly with the atomic electrons, and these in turn can couple strongly with the nucleus.
Nature has been kind in providing a “laboratory” to study such interactions in the form of a nucleus, thorium-229, that has an isomer with an excitation energy of only 3.5 ± 1.0 eV. (This error bar is rather large because the energy is measured as the difference of two, much larger, numbers.) Indeed, this excitation energy is so low that it is less than the ionization energy of thorium. Moreover, the 3.5 eV isomer of thorium-229 has a half-life of 45 hours and is conveniently produced in the alpha decay of uranium-233.
During the last couple of years, the race has been on to detect the ultraviolet photons, termed “gamma-rays” on account of their nuclear origin, that would signal the de-excitation of the 3.5 eV isomer. Indeed, two groups reported recently that they had observed this process. However, the latest results suggest that the signal was actually due to alpha-particle-induced fluorescence of nitrogen in the air surrounding the samples (S B Utter et al . 1999 Phys. Rev. Lett . 82 505; R W Shaw et al. 1999 Phys. Rev. Lett . 82 1109). It is not possible to dispense with the alpha particles, unfortunately, because they come from the uranium-233 decays that are used to produce the thorium-229 isomer in the first place.
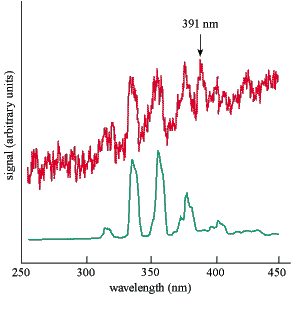
However, all is not yet lost. Robert Shaw and co-workers at the Oak Ridge National Laboratory in Tennessee have made a detailed comparison of the ultraviolet spectrum from uranium-233 in air with the corresponding emissions from a nitrogen discharge (see right). The strongest transitions in both samples are very similar, but there is an additional transition from uranium-233 in air, at 391 nm (3.2 eV), that remains unexplained. Shaw and colleagues are cautious, saying that “it could be the thorium-229 ultraviolet gamma-ray”, but adding that there are “other candidates for its identity”.
Although not discussed by the Oak Ridge group, it is evident that if the 391 nm transition can be identified with the spontaneous decay of the 45-hour thorium-229 isomer, then the reverse process could be attempted: that is it should be possible to excite the nucleus from its ground state into the isomer with a 391 nm laser beam. And if nuclei can be “tickled” with a laser beam, then it might be possible to harness the huge energy density of highly excited isomers.
So, who will have the last laugh? The thorium-229 story is far from over, and the hafnium-178 story has only just begun. In both cases the isomers serve as “laboratories” for exploring the interactions of low-energy photons with atomic nuclei. Taken in combination with the new laser-fusion results it is apparent that the impact of light on nuclei has a bright future.



