Shining laser light through the skin can be used to form detailed images of tissues just below the surface, as a way to diagnose disease. High-power lasers can also be used therapeutically to disrupt diseased regions of tissue. A group at the Nello Carrara Institute of Applied Physics in Italy has developed and tested a new type of nanoparticle that could be introduced into tissue to react to the laser light more efficiently – and improve the effectiveness of these techniques (J. Biophotonics 10.1002/jbio.201900082).
One technique that the group hopes to use with the nanoparticles is photoacoustic imaging. This uses a short laser pulse to focus light into a precise region of tissue, up to a centimetre below the surface of the skin. The energy is absorbed and the region heats up a little, which causes it to vibrate and emit a small ultrasound pulse that can be picked up by a detector on the surface.
The magnitude of the emitted ultrasound is dependent upon the tissue properties, enabling different structures to be identified. Photoacoustic imaging can visualize much deeper tissues than imaging with laser light alone. This is because the light only has to travel one way through the tissue; the returning ultrasound waves are not scattered as much and can pass through the body far more easily. Nanoparticles that respond to the laser light might make photoacoustic imaging more effective.
Laser-stimulated gold nanoparticles have also previously been developed as a way to destroy cancerous cells. The nanoparticles can be coated with a compound that enables their absorption by specific cells. The particles respond to an applied laser light pulse and heat up their surroundings, killing the cells in a targeted way. Such nanoparticles have not been used clinically, however, either because of toxicity concerns or because they don’t respond well enough to wavelengths of light that penetrate deep into the body.
In their latest study, the researchers produced gold nanorods, around 75 nm long and 15 nm wide, and coated with polyethylene glycol and a peptide to reduce any negative biological effects and encourage their uptake by cells. They tuned the size of these nanorods to absorb infrared laser light at a wavelength of 1064 nm.
Previously, nanorods have only been able to absorb light of around 800 nm, which is less suited to medical applications, due to restrictions on maximum laser intensity at shorter wavelengths, and greater availability of longer wavelength lasers. These nanoparticles are also designed to respond to laser stimulation by producing bubbles, which break apart the cells, rather than destroying them through extreme heating.
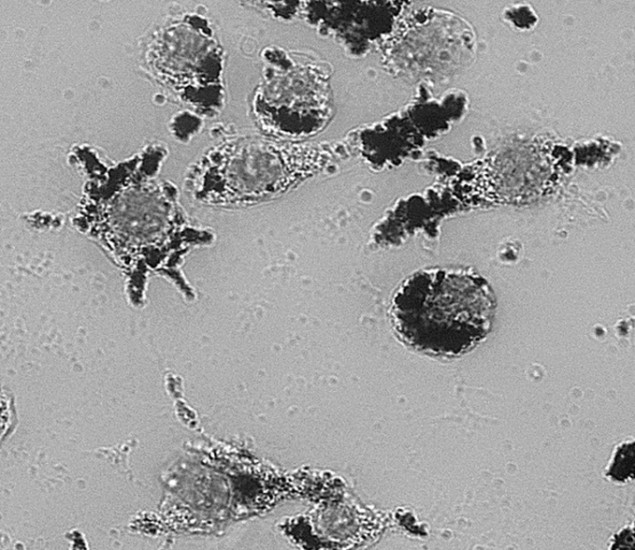
The team tested their nanoparticles on isolated macrophages (white blood cells) and saw that the rate at which they were taken up was similar to previous types of nanoparticles. The nanoparticles did not appear to be toxic to the cells once absorbed, and no signs of apoptosis were observed. When the researchers applied laser pulses, the particles produced microbubbles within the cells that emitted detectable ultrasound waves. The bubbles were also able to cause cell death at laser intensity levels currently permitted under clinical guidelines.
“For clinical translation, I think that the use of nanoparticles to impart localized therapies is the most interesting, with the advantage of imaging support,” says lead author Lucia Cavigli. “Superficial cancers such as skin cancer, or cancers with easy access via an optical fibre (such as bowel cancer) would be particularly suitable for this approach.”
Further tests are needed to assess the safety of the nanorods, and how well they would function within the body. In particular, it’s important to see how long the particles remain in the body after they have been injected, to find out whether and how they are broken down and excreted.



