Tissue engineering offers the potential to improve treatment of injured or diseased bone, by using stem cell-laden biomaterials that promote tissue repair, for example. Three-dimensional bioprinting of such biomaterials can fabricate complex scaffolds that mimic bone in composition and can be customized to a patient’s particular bone defect. Developing the optimal bioink to fabricate these living implantable constructs, however, remains highly challenging.
Researchers in Portugal have now developed a nanocomposite bioink containing bioactive materials that instruct stem cells to change into bone cells. They demonstrated successful printing of their bioink into stem cell-laden constructs, reporting their findings in Biofabrication.
The distinctive feature of this new bioink is that it contains both the organic and inorganic constituents of bone. “In nature, the building blocks of bone tissue include organic biomolecular and inorganic nanostructured functional elements,” explains senior author João Mano, leader of the COMPASS research group at the University of Aveiro. “The nanocomposite bioink recapitulates these components.”
For the organic component, the team chose gelatin methacrylate (GelMA) – a collagen derivative used for 3D bioprinting that exhibits adjustable mechanical properties and biocompatibility. The gelatin also contains functional groups that can attach to cells, such as stem cells.
For the inorganic part, the researchers used mesoporous silica nanoparticles functionalized with calcium, phosphate and dexamethasone (MSNCaPDex). Calcium and phosphate ions positively influence bone matrix deposition and mineralization, as well as encouraging osteogenic differentiation (where the stem cells develop into bone cells). Dexamethasone, meanwhile, also induces such osteogenic differentiation. They completed their bioactive bioink by loading the GelMA/nanoparticle combination with human bone marrow-derived mesenchymal stem cells (hBM-MSCs).
Proving printability
In a first proof-of-concept step, Mano and colleagues demonstrated that they could print stable 3D constructs using GelMA. They employed 3D extrusion bioprinting to create small discs from 10% solutions of GelMA prepared at 37 °C and then cooled to increase viscosity. They found that incubating the GelMA on ice for 5 min, then printing at a pressure of 65 kPa and speed of 10 mm/s created 3D constructs with highly defined shapes.
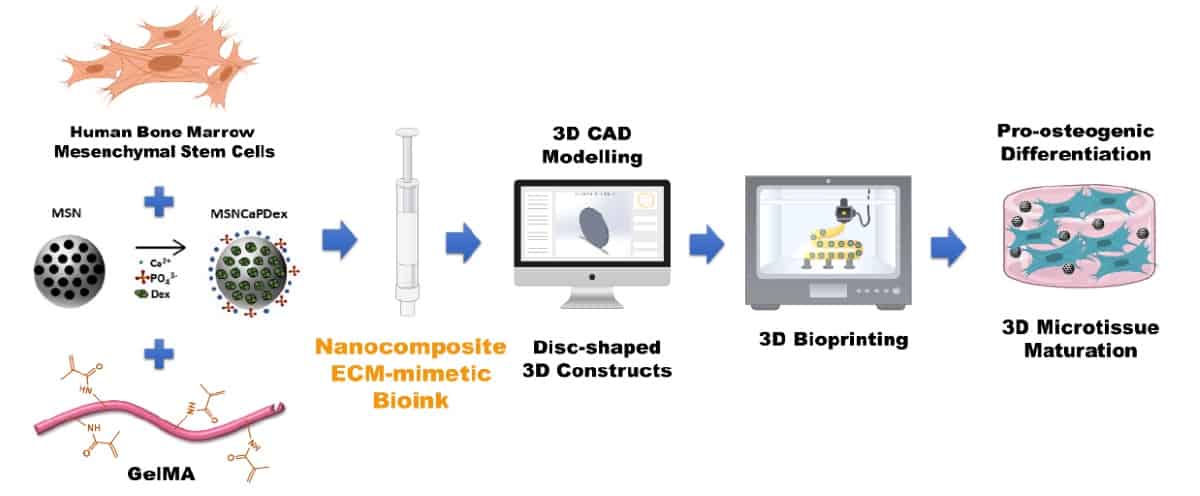
After optimizing the GelMA printing process, the researchers added MSNCaPDex (0.5%) to create the nanocomposite bioink. The bioactive nanoparticles should release calcium, phosphate and silicate ions, all of which are involved in bone repair. To test this, they assessed the bioactivity of printed GelMA discs with and without nanoparticles, after three days in simulated body fluid.
In the nanocomposite hydrogel discs, energy dispersive spectroscopy (EDS) mapping clearly showed the presence of silica, calcium and bone-like calcium phosphate apatite. Conversely, only traces of calcium and phosphorous were seen in plain GelMA discs, with no structures resembling apatite. FTIR spectroscopy and X-ray diffraction confirmed the mineralization activity of the nanocomposite hydrogel.
“The existence of deposited hydroxyapatite in the bioink is highly valuable since it is a key component of native living bone tissues,” says Mano. “Its presence is known to have a positive influence on bone regeneration, particularly upon constructs implanted in vivo, since it allows the establishment of a bone–implant interface where de novo deposited bone binds to hydroxyapatite, hence improving osteointegration.”
Next, the researchers assessed the viability of the stem cells after bioprinting. They observed that the stem cells remained viable after two weeks of culture, with stable metabolic activity and DNA content for 21 days. These results show that neither 3D bioprinting nor encapsulation in GelMA affected the cells’ viability.
Bone generation
The other function of the bioactive nanoparticles is to induce the stem cells to differentiate into bone cells. To assess this property after bioprinting, the team examined the behaviour of stem cells within GelMA incubated in basal medium (negative control), GelMA in osteogenic medium (positive control) and GelMA/MSNCaPDex in basal medium.
The researchers evaluated two biomarkers involved in bone formation: bone morphogenetic protein (BMP-2) and osteocalcin. After 14 days, BPM-2 levels were significantly higher for stem cells in the nanocomposite hydrogel than in either control. After 21 days, the BMP-2 level in the positive control was similar to that of the bioprinted nanocomposite. The positive control and nanocomposite hydrogels exhibited similar levels of osteocalcin, both higher than that of the negative control.
“Owing to the intrinsic bioactivity and bioinstructive features of ion/drug-loaded silica nanoparticles, their inclusion in GelMA enabled a guided differentiation of mesenchymal stem cells towards osteoblasts, the cells that naturally build bone, without requiring further synthetic supplementation that is usually necessary for this process,” Mano tells Physics World.
The researchers are now exploring the fabrication of more morphologically complex constructs using this new bioink. “We are also using more advanced 3D bioprinting technologies such as suspension bioprinting using a viscoelastic supporting bath,” says Mano. “This allows truly freeform bioprinting and the manufacture of higher-order nanocomposite bioink-based constructs.”