Graphene nanostructures with zigzag-shaped edges show much technological promise thanks to their excellent electronic and magnetic properties. Unfortunately, the highly reactive edges of these so-called graphene nanoribbons (GNRs) degrade quickly when exposed to air, limiting their practical applications. A team in Spain and the Czech Republic have now come up with two new strategies for protecting them. These strategies could also be extended to other types of technologically important carbon-based nanostructures.
GNRs are special because the behaviour of their electrons can be tuned from metal-like to semiconducting simply by adjusting the length or width of the ribbons, modifying the structure of their edges or doping them with non-carbon atoms. The materials can also be made magnetic using these techniques. The versatility of GNRs makes them promising building blocks for numerous applications, including quantum technologies.
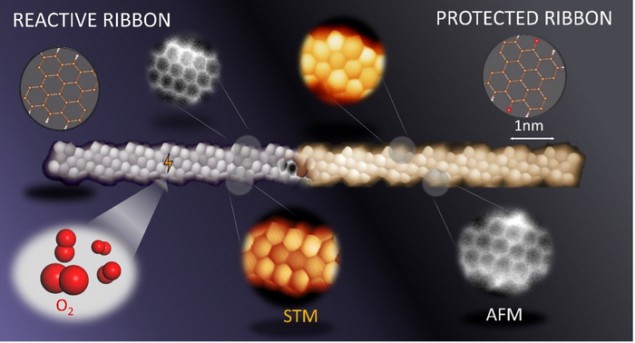
The problem is that the exceptional properties of GNRs rely on the presence of zigzag-shaped segments along their edges, and these segments (unlike armchair-shaped edges) are unstable in air. This means that GNRs need to be kept in vacuum, making it hard to employ them in real-world applications.
sp3 configuration increases air stability
In the new work, three research groups – led by Dimas G de Oteyza of the Nanomaterials and Nanotechnology Research Center (CINN) in El Entrego, Spain; Diego Peña from CiQUS, Universidade de Santiago de Compostela; and Pavel Jelinek at the Institute of Physics, Czech Academy of Sciences – studied narrow strips of graphene nanoribbons with a large density of zigzag-shaped edges. They found that when hydrogenated, the carbon atoms in the nanostructures rehybridize into a sp3 configuration, which increases their stability in air. The structures can be converted back to their original state simply by heating them up. Alternatively, the researchers found that they could make the nanostructures stable by functionalizing them with ketone side-groups. This oxidized form of the material is stable to a variety of other chemicals, too, and can be converted back to the pristine form by hydrogenation and annealing under vacuum conditions. In both cases, the protected GNRs retain the electronic properties of the pristine nanostructures.
“Our protection strategies allow us to take these molecules out of the inert vacuum environment without degrading them,” Oteyza tells Physics World. “These techniques may be extrapolated to different GNRs and carbon-based nanostructures, as well as to different functional groups, allowing these zigzag-edged carbon materials to be used in scalable real-world applications.”

Squashed carbon nanotubes make smooth nanoribbons
Before this becomes possible, however, Oteyza and colleagues acknowledge there are challenges to overcome. “For one, the ‘deprotection’ steps still require vacuum conditions,” explains Peña. “This means that while we can place our molecules of interest into the appropriate device structures for scalable applications, the devices must still work in vacuum.”
An additional step will therefore be required, namely protecting the structure of the whole GNR-based device in a way that does not affect the molecule’s chemistry. “This is one of the main challenges that we need to tackle,” Jelinek says.
The study is published in Nature Chemistry.