Techniques that exploit the phase of X-rays, not just their absorption, have the potential to transform X-ray imaging in hospitals, reports Jude Dineley

“I was completely blown away,” says Alessandro Olivo of University College London (UCL) in the UK, recalling the fine detail of a wasp revealed in his first X-ray phase-contrast image. Then working at the University of Trieste in Italy, Olivo and colleagues took the image in 1997 at Elettra, a synchrotron on the outskirts of the city. The research was part of a global resurgence of interest in X-ray phase-contrast imaging (XPCi) in the 1990s led by Japanese pioneer Atsushi Momose.
The first XPCi image was acquired by Ulrich Bonse and Michael Hart at Cornell University in New York in 1965. The renewal in interest was motivated by the technique’s potential to improve on conventional X-ray images, in medicine as well as in other applications.
Introduced to hospitals shortly after their discovery by Wilhelm Röntgen in 1895, X-rays’ ability to non-invasively peer inside a patient rapidly made them indispensable. Today, 3D computed tomography (CT) and real-time fluoroscopy (which uses X-rays to obtain real-time moving images of a patient’s internal anatomy) have also become invaluable. The underlying physical principle, which is to create images based on the fact that different materials in the body attenuate X-rays to different extents, has remained unchanged in 120 years. However, this approach hides subtle composition fluctuations in organs and other soft tissues. Refraction at an interface – a manifestation of phase change – is a significantly more sensitive indicator of composition. In fact, the resulting variations in speed are up to 1000 times greater than changes in attenuation.
Better images and lower doses
The potential implications for medicine of the XPCi technique are profound. It could improve the detection and characterization of abnormalities. Alternatively, increased image sensitivity could be traded for shorter exposures and lower radiation doses, reducing a small but finite risk of radiation-induced cancer.
Increased image sensitivity could be traded for shorter exposures and lower radiation doses, reducing a small but finite risk of radiation-induced cancer
In imaging the breast – a radiosensitive soft-tissue structure – mammography arguably has the most to gain from XPCi and is attracting a lot of attention from researchers. Lower doses in cancer screening and improvements in image quality could have large health and economic benefits, particularly for the majority of people who do not have cancer. Currently, absorption-based mammography returns around nine false-positive results for every tumour correctly identified, resulting in unnecessary and invasive follow-up investigations.
The simplest XPCi technique is free-space propagation. It involves firing a highly coherent beam through an object, which changes its phase and refracts it by angles of the order of 1 μrad – equivalent to a 1 mm deflection 1 km from the object. X-rays adjacent to the object provide an unshifted reference that combines with the refracted X-rays to generate an interference pattern. Unlike absorption-based imaging, where the detector is placed directly behind the object, a detector recording the pattern is placed between tens of centimetres and several metres downstream where the interference pattern can be resolved.
Highly coherent, intense and energy-tunable synchrotrons provide perfect beams for free-space propagation and other XPCi techniques. Starting in 2006, researchers co-led by Renata Longo of the University of Trieste undertook the first clinical XPCi synchrotron study. Using free-space propagation with monochromatic 17–22 keV X-rays at Elettra, they imaged 71 women for whom conventional mammography and ultrasound returned inconclusive findings.
Despite the tough challenge the cohort posed, the images were better than conventional mammograms and doses were comparable or lower. Excitingly, XPCi also cleared 17 individuals subsequently confirmed as tumour-free where absorption imaging had failed. “We have a technique that has the potential to decrease the number of healthy people undergoing further examinations,” says Longo.
From synchrotron to lab
However, before all patients can benefit from XPCi, compact, robust and affordable techniques are needed. The only commercial system, which was launched by Japanese company Konica Minolta in 2005, has so far had limited impact, says Olivo. A partially coherent X-ray source combined with a free-space propagation approach restricted improvements in image contrast over conventional X-ray images.
An alternative approach that sidesteps the requirement for beam coherence is edge illumination, which measures how much X-rays get deflected when refracted by an object. Developed by Olivo, the method uses two “masks”: one in front of the X-ray source and another in front of the detector. Each comprises a set of apertures with a period – or slit spacing – of around 70–80 μm, the first creating a set of beamlets.
During a reference scan with no object present, the masks are aligned so that only half of each beamlet passes through an aperture onto a detector pixel. When an object is introduced, more or less of the beamlet hits the pixel, depending on its deflection. The measured intensity change can be used to derive the phase change.
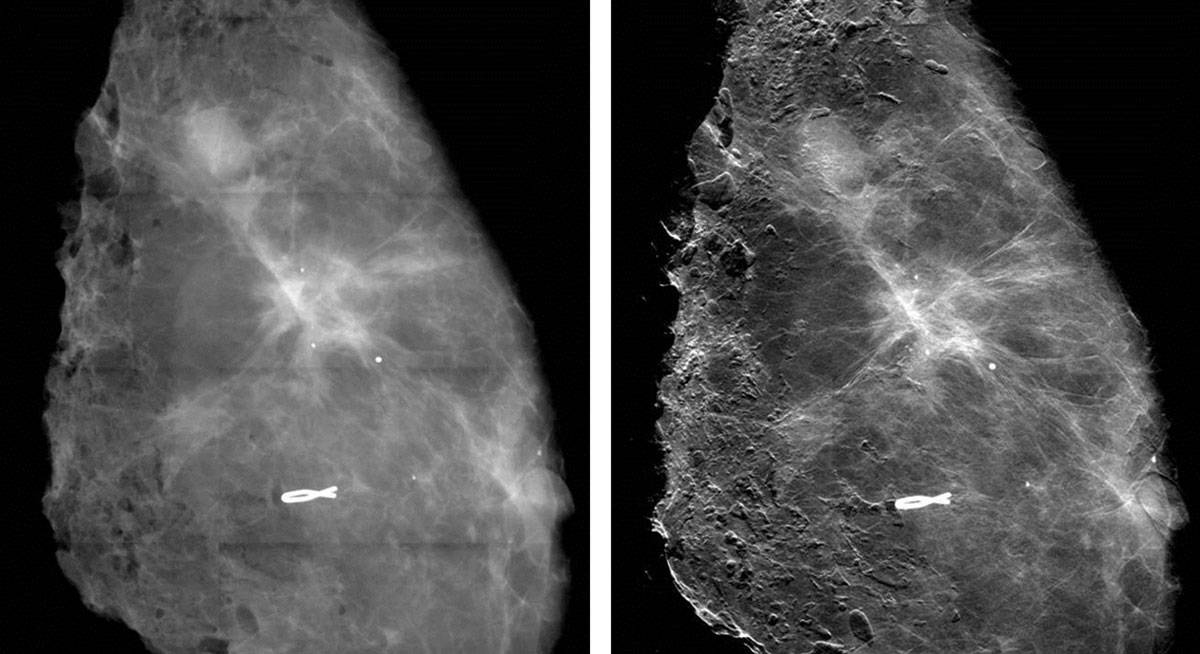
The latest mammography prototype at UCL uses a powerful polychromatic X-ray source that enables quick exposures and results in breast doses comparable to those in clinical practice. The masks are easy to make in larger sizes, enabling extended fields of view. Imaging excised breast tissue, the system has detected features invisible in absorption images and increased the contrast of “microcalcifications” – tiny deposits that can indicate cancer – by up to a factor of nine.
For manufacturers, a potential limitation is that the devices have to be quite tall because the further the X-rays travel from the patient, the more they get deflected and the more sensitive the system is. In fact, standing 1.5 m high, the UCL system may be too large to be incorporated into existing commercial systems.
Another technique – grating-based interferometry – involves placing a diffraction grating in front of a regular polychromatic X-ray tube to increase coherence. Originally developed in synchrotrons, it was first achieved by Franz Pfeiffer and colleagues at the Paul Scherrer Institute (PSI) in Zurich, Switzerland. Pfeiffer now leads a group at the Technical University of Munich (TUM) in Germany.
X-rays transmitted through an object pass directly through a second grating that creates a diffraction pattern downstream at a specific distance. A third “analyser” grating in front of a detector interrogates the pattern. The phase shifts can be deduced by comparing the intensities with a reference scan with the object absent.
Imaging challenges
Researchers are tackling several issues that dictate the performance of grating-based XPCi. In general, the X-rays needed to image structures deep in the body are several times more energetic than those that have been used to date in XPCi studies of the breast, tissue samples and laboratory animals. Higher energies require gratings thick enough to absorb X-rays incident between slits that would otherwise degrade the diffraction pattern. However, high-sensitivity imaging requires a small period and fabricating a stable grating with both properties is difficult.
Several groups are beginning to overcome this challenge. Using gratings made by researchers at the Karlsruhe Institute of Technology in Germany, a collaboration led by Pfeiffer has acquired 82 keV and 133 keV images – the highest to date – at the European Synchrotron Radiation Facility in Grenoble, France. “Grating fabrication has improved such that gratings with high aspect ratios – structures up to 150 μm high with very small periods down to 2.4 μm – are now possible,” says Julia Herzen, who is leading research at TUM that aims to translate advances in the lab into clinical applications.
By absorbing X-rays, gratings also reduce the number of photons reaching the detector and are a complicating factor in the trade-off between image sensitivity and dose. The challenge is to identify the sweet spot that improves contrast beyond that of absorption images, yet keeps patient doses within prescribed limits.
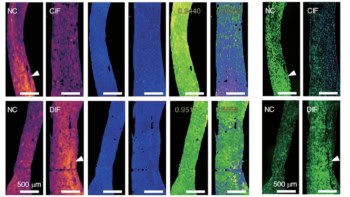
With simultaneous absorption, phase-contrast and dark-field images of excised breast tissue, a Swiss Federal Institute of Technology Zurich and PSI group led by Marco Stampanoni is using grating-based imaging to provide further evidence of the clinical potential of phase measurements. In a first for the technique, the researchers demonstrated significant improvements in image quality in the lab.
Dark-field imaging is a variation of the phase-contrast principle and is a hot topic in the XPCi community. Instead of detecting phase shifts that highlight boundaries between macroscopic objects, it detects ultra-small-angle scattering by microscopic structures. Pioneered in the early 2000s, dark-field imaging can be achieved with the same pixelated detectors used for XPCi. “This is a very important new signal because you can get information from structures that are much smaller than pixels in a coarse detector,” says Stampanoni.
In a separate study, the group has taken the first steps towards a quantitative measure that could help assess the risk of cancerous or precancerous cells in clusters of microcalcifications in the breast. Analysing microcalcifications in mastectomy samples, the researchers found that some scattered a lot yet absorbed few X-rays, while others exhibited the opposite characteristics. Stampanoni’s team hypothesizes that the ratio of the two signals could discriminate between two types of microcalcification: one rarely associated with cancer and another commonly found in so-called proliferative lesions that include the most aggressive breast cancers.
However, more work is needed. As all of the tissue came from patients with breast cancer sufficiently advanced to require mastectomies, the sample was biased. “We have an indication where this threshold could be, but we need to have much stronger statistical evidence,” says Stampanoni.
From the lab to the hospital
The only company with an imager in a hospital is Konica Minolta, which is working with researchers at Japanese universities, including Momose. In a simple application, its second, grating-based prototype system has imaged the finger joints of volunteers in 2D, using a small field of view and mean beam energy of 28 keV, where each image takes tens of seconds to acquire. There are plans to adapt the system for mammography.
One long-term goal for the XPCi community is 3D imaging in patients. A large Italian consortium co-led by Longo is planning the first patient scans in a second breast-imaging study at Elettra, which is due to start by the end of 2016. With the ideal conditions that the synchrotron provides, the study will give a performance benchmark for phase-contrast breast CT. Like conventional CT, it requires multiple projections taken over 360° around the patient. Achieving this without a synchrotron, within non-negotiable dose limits and in short exposures in particular, is an even tougher challenge than for 2D imaging.
While several groups have links with companies, aside from the newer Konica Minolta prototype there is no 2D or 3D commercial imager on the horizon, making it hard to predict when patients might start benefiting from XPCi. Funding is a big issue because XPCi has reached a stage where it is no longer eligible for blue-sky funding from research councils, yet is not advanced enough to gain serious financial support from business, which means that – according to Olivo – groups might struggle to find the funding needed to push XPCi into the clinic. “You can make anything work if you throw enough money at it, once you have the proper principle,” he says. “I cannot build a perpetual motion machine, but I think I can build a phase-contrast imager!”
Beyond mammography

Researchers around the world are using phase-contrast X-ray imaging to study many different diseases and areas of the body, including cartilage, bone, lungs, blood vessels and kidneys, typically using animals and tissue samples because human scanners are still under development.
In the lungs, the interface between air and soft tissue provides the strongest phase gradient in the body. Potential applications include diagnosing and monitoring conditions that impair lung function, like emphysema and cystic fibrosis. With multiple overlapping airways, the lungs appear in phase-contrast images as speckled masses that are hard to read by eye, and research has focused on quantitative analyses.
Examining absorption and dark-field signals in the lungs of live mice using a miniature CT scanner developed in-house, researchers at the Technical University of Munich measured dramatically lower scattering signals in emphysematous lungs, while conventional imaging showed little change.
Marcus Kitchen’s group at Monash University in Australia, meanwhile, has developed techniques that quantify lung function by analysing the speckle texture. “Our technique uses a statistical approach to say what is the average size and number of the airways that are contributing to the speckle pattern,” he explains.
Using animal models, the researchers have employed XPCi to identify optimal approaches for the resuscitation of newborn babies with great success. Their findings have resulted in changes to clinical practice around the world.
Also at Monash, Kaye Morgan and colleagues were the first to measure the depth of fluid lining the airways in live mice, using a high-speed XPCi technique they developed. Thinner fluid layers in people with cystic fibrosis make them more prone to infection, and the researchers are using the technique to investigate new therapies. On the physics side, the group is working on approaches that could enable monitoring of individual patients. “It’s definitely a long-term goal,” says Morgan.