The brain’s frontal circuits play a vital role in controlling motor, cognitive and behavioural functions. Disruption of the fronto-subcortical circuits, which connect the frontal cortex in the forebrain with basal ganglia located deeper within, can result in a range of neurological disorders. It’s not clear, however, which connections are associated with which dysfunctions. To shed light on this problem and help identify potential treatment targets, an international research team has used deep brain stimulation (DBS) to map the circuits associated with four different brain disorders.
DBS is an invasive therapy in which surgically implanted electrodes modulate brain networks by electrical stimulation of target regions. One such target – the subthalamic nucleus – is of particular interest as it receives input from the entire frontal cortex to the basal ganglia. Indeed, electrical stimulation of the subthalamic nucleus has been shown to alleviate symptoms of several brain disorders.
The research team – led by Andreas Horn from the Center for Brain Circuit Therapeutics at Harvard Medical School and Charité – Universitätsmedizin Berlin, and Ningfei Li from Charité – studied a total of 534 DBS electrodes implanted to treat four brain disorders: Parkinson’s disease (PD), dystonia, obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS).
First author Barbara Hollunder and colleagues first examined data from 197 patients who had DBS electrodes bilaterally implanted in the subthalamic nucleus to treat these disorders, including 70 with dystonia, 94 with PD, 19 with OCD and 14 with TS.
For each disorder, they mapped stimulation effects at the subthalamic level across the cohort to identify the sites associated with the most beneficial stimulation. These DBS “sweet spots” differed in location on the subthalamic nucleus for the four disorders.

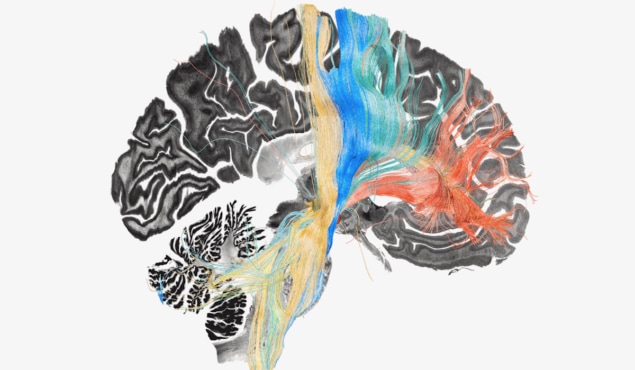
Next, the researchers mapped stimulation effects to the fronto-subcortical circuits, enabling them to identify which brain circuits had become dysfunctional (and could be targeted for treatment) in each disorder. The circuits that benefitted most from stimulation (referred to as “sweet streamlines”) included projections from sensorimotor cortices for dystonia, the primary motor cortex for TS, the supplementary motor area for PD, and the ventromedial prefrontal and anterior cingulate cortices for OCD.
“We were able to use brain stimulation to precisely identify and target circuits for the optimal treatment of four different disorders,” says Horn in a press statement. “In simplified terms, when brain circuits become dysfunctional, they may act as brakes for the specific brain functions that the circuit usually carries out. Applying DBS may release the brake and may in part restore functionality.”
Clinical potential
These disease-specific streamline models hold potential for guiding future clinical treatments. To confirm this capability, the researchers performed further experiments using independent data. They validated the PD and OCD streamline models (selected due to patient availability) in two additional retrospective groups of 32 and 35 patients, respectively.
In these additional patients, the researchers used the level of overlap between stimulation volumes and the respective streamline model to estimate clinical outcomes. For both disorders, they observed a good match between the estimates and improvements in symptoms.
The researchers also performed three prospective experiments using the identified circuits to improve treatment benefit. For two patients, they reprogrammed their DBS implants to maximize the overlap of stimulation volumes with the respective streamline model. The first patient, a 67-year-old male with PD, had benefited from a 60% reduction in symptoms upon conventional clinical treatment with DBS. Optimized stimulation based on streamline-guided parameters improved this treatment benefit further to a 71% reduction in symptoms.
In the second case, a 21-year-old female with severe treatment-resistant OCD, one month after streamline-based DBS reprogramming she experienced a 37% reduction in global obsessive-compulsive symptoms, compared with a 17% symptom reduction under clinical stimulation parameters.
Finally, the team implanted a pair of subthalamic electrodes to treat a 32-year-old male who had suffered from treatment-resistant OCD since the age of 18. Four weeks after surgery, with DBS informed by the streamline models, he reported a 77% reduction in global obsessive-compulsive symptoms, with improvements seen within one day of switching on the DBS.
Personalized brain stimulation could treat untreatable depression
The researchers suggest that their successful validations of the OCD and PD streamline targets may provide initial evidence for clinical applications in the context of prospective validation studies. They note that – if further confirmed – the identified circuits may represent therapeutic targets that could also be used for stereotactic targeting in neurosurgery and potentially non-invasive transcranial magnetic stimulation.
Li tells Physics World that in future, the researchers “plan to refine the model, focusing more on fine-grained dysfunctional brain circuits, and validate our findings through prospective clinical trials”.
The researchers describe their findings in Nature Neuroscience.




