Contrast-enhanced ultrasound delivered using microbubbles can readily differentiate between different brain regions and structures, as well as various types of tumours, researchers from Italy and the US have reported. The findings of the study – which the team says is the first large-scale quantitative analysis of microbubble-enhanced cerebral ultrasound images – could open up new avenues for ultrasound-based imaging and therapies.
Formed by encapsulating gas within a flexible lipid, polymer or protein shell, microbubbles, as their name suggests, have diameters in the range of one-to-four microns. When injected into the body intravenously and then subjected to a sonic field, these tiny bubbles vibrate and collapse, producing distinctive harmonic signals that can be used to distinguish them from surrounding tissues, allowing their use as an ultrasound contrast agent.
In addition, when combined with focused ultrasound, microbubbles can be used to produce various biological effects, including reversibly opening the blood–brain barrier and easing drug delivery. But they also come with the risk of cavitation-induced tissue damage. Understanding how microbubbles end up distributed within the body is therefore important for both accurate imaging and safe therapeutic applications. For the brain, however, such knowledge has been somewhat lacking.
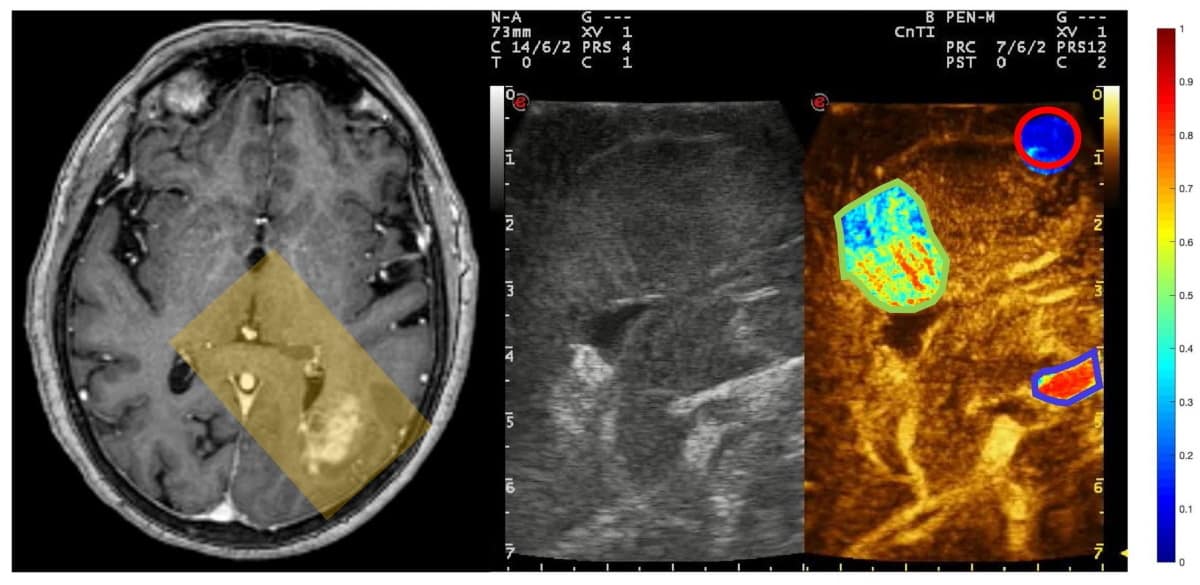
To address this, neurosurgeon Francesco Prada of the Fondazione IRCCS, Istituto Neurologico Carlo Besta in Milan and his colleagues performed contrast-enhanced ultrasound imaging of the brains of 19 patients while they were undergoing surgery to remove tumours. Following each procedure, they quantified and compared the changing distribution of microbubbles across the target area for key structures – specifically, arteries, tumours and white matter, as mapped by a trained neurosurgeon.
The researchers found that there were clear differences in microbubble distribution across the brain, with arteries showing the earliest and largest enhancement of the structures studied, followed by tumours and then regions of white matter. Specifically, the team’s analysis revealed that artery, tumour and white matter structures had peak microbubble intensities of 0.584, 0.436 and 0.175 units, respectively.
“Understanding cerebral microbubble distribution holds tremendous potential to improve both image guidance during surgical procedures and planning ultrasound-mediated therapies,” Prada tells Physics World. “Performing quantitative analysis of such phenomena will provide a sharper understanding and also will allow data sharing and ensure replicability of the results,” he explains, noting that such work will help pave the way towards safer procedures for patients.
“Performing brain surgery allows one to literally ‘open’ an acoustic window to the brain,” Prada adds. “This is an opportunity that should be exploited to its best, not only to improve a single procedure, but to gather pivotal data that will change our understanding of how the brain and its diseases function.”
“This work nicely demonstrates how ultrasound imaging in conjunction with microbubbles injection provides quantitative and highly informative parameters on different brain tissue,” comments Mickael Tanter, a medical physicist from the French National Institute of Health and Medical Research who was not involved in the present study. “The ability of contrast-enhanced ultrasound imaging to discriminate white matter, parenchymal tissue and intra-tumoural tissue with portable imaging scanners will surely make ultrasound the method of choice for per-operative imaging during brain surgery in the coming years.”
Ultrafast ultrasound maps tiny blood vessels deep in the human brain
Jens Eyding – a neurologist from the Gemeinschaftskrankenhaus Herdecke in Germany, who was also not involved in the present study – notes that contrast-enhanced ultrasound imaging of the brain has long been regarded as having potential for use during tumour resection, especially given that shifts in brain mass following a craniotomy can render inaccurate surgical guidance derived from a pre-operative MRI scan.
“I absolutely agree on the need for more research on perfusion patterns of different brain tissue,” he says. “A prospective and systematic study to characterize specific parametric perfusion patterns of different tissue types may help to improve the significance of surgical guidance by means of ultrasound.”
With their initial study complete, the researchers are now looking to explore the potential of contrast-enhanced ultrasound-guided and microbubble-mediated therapies as a cheaper alternative to MR-guided focused ultrasound. Prada has also patented a cranial implant that can allow transcranial direct ultrasound imaging and therapy.
The study is described in Scientific Reports.