
Spheroids – three dimensional “balls” of cultured cells – are widely used within medical research and increasingly employed as building blocks for tissue engineering applications. Current methods for creating these spheroids, however, are time-consuming, require large amounts of reagents and have high running costs.
A research team in Austria has now developed a versatile and cost-effective technique for high-throughput spheroid production, using compartmented cell-culture dishes. Writing in Biofabrication, the team explains how the new system considerably reduces production costs and time, and can create spheroids with potential for use in tissue regeneration.
The researchers used laser engraving to quickly and easily create grids on standard 60 mm cell culture dishes. Each dish can produce approximately 200 spheroids, with one spheroid growing in each compartment.
“In previously work, we observed that growth surface restriction can cause focal cell aggregations. Realizing the potential for 3D culture generation, we developed a system based on compartmented culture plates,” explains Sylvia Nürnberger from Medical University of Vienna. “On these surfaces, mesenchymal stromal cells and chondrocytes not only aggregated, but formed complete spheroids that eventually detached from the surface. This method of spheroid formation is completely new.”

The novel production method requires less handling time than standard pellet culture approaches, in which spheroids are grown in a 96-well plate, and uses 10 times less media, significantly reducing reagent costs. “Our system also allows for additional early read-outs, since the spheroid formation phase by itself provides additional information on the cellular fitness,” adds first author Marian Fürsatz.
Spheroid growth kinetics
Nürnberger and colleagues studied spheroid formation using human adipose-derived stem cells (ASC/TERT1) and human articular chondrocytes (hAC) – cells with potential for use in cartilage repair.
When seeded in the grid plate, both cell types adhered only to the compartment surface (not to the laser incisions), where they initially formed a 2D cell monolayer. Spheroid formation started with contraction of this monolayer at the compartment edges, followed by rolling up of the cell layer, typically leaving a few points anchored to the plate surface. Conversion into spheroid form involved rapid loss of an anchor point, contraction and then full condensation into a sphere.
The stem cells formed free-floating spheroids after approximately three weeks, the chondrocytes after two months or more. By changing the grid size, the researchers could control the final spheroid size. Growth on 1 and 3 mm grids resulted in ASC/TERT1 spheroids with mean diameters of 134 and 340 µm, respectively.
As the stem cells created spheroids faster than the chondrocytes, the researchers tested whether adding ASC/TERT1 to hAC cultures could speed up their formation. Surprisingly, they found that the co-cultures formed spheroids even faster than ASC/TERT1 cells alone.
Assessing different ASC/TERT1:hAC ratios (80:20, 50:50 and 20:80) revealed that co-cultures with 50:50 and 20:80 ratios formed spheroids in roughly seven and six days, respectively, significantly faster than pure ASC/TERT1 cultures, which took around 21 days. The co-cultures created spheroids with slightly lower diameter, due to their faster formation, with higher hAC ratios causing greater size variation and more small spheroids.
Next, the researchers compared the differentiation capacity of spheroids generated by grid plates and via standard pellet culture. The relative gene expression and differentiation index were comparable between grid-plate and pellet culture spheroids. Both production methods created spheroids with similar circularity and roundness.
Comparing the internal structure of grid-plate spheroids and standard pellet cultures revealed clear differences in their internal structure. In particular, grid-plate ASC/TERT1 spheroids exhibited internal strands of dense matrix. These compact matrix strands might provide a denser and more cartilage-like environment for the cells and a higher stiffness, which could be an advantage for in vivo application.
Tissue regeneration potential
The researchers next assessed the suitability of grid-plate spheroids for cartilage repair, by embedding ASC/TERT1 and 50:50 hAC:ASC/TERT1 spheroids into a fibrin hydrogel. Co-cultures showed strong cellular outgrowth in all directions, reaching an approximate radius of 0.5 mm after 14 days. ASC/TERT1 spheroids showed higher outgrowth towards other spheroids, but slower outgrowth in other directions. Staining revealed that co-cultures induced greater matrix deposition around the spheroids.
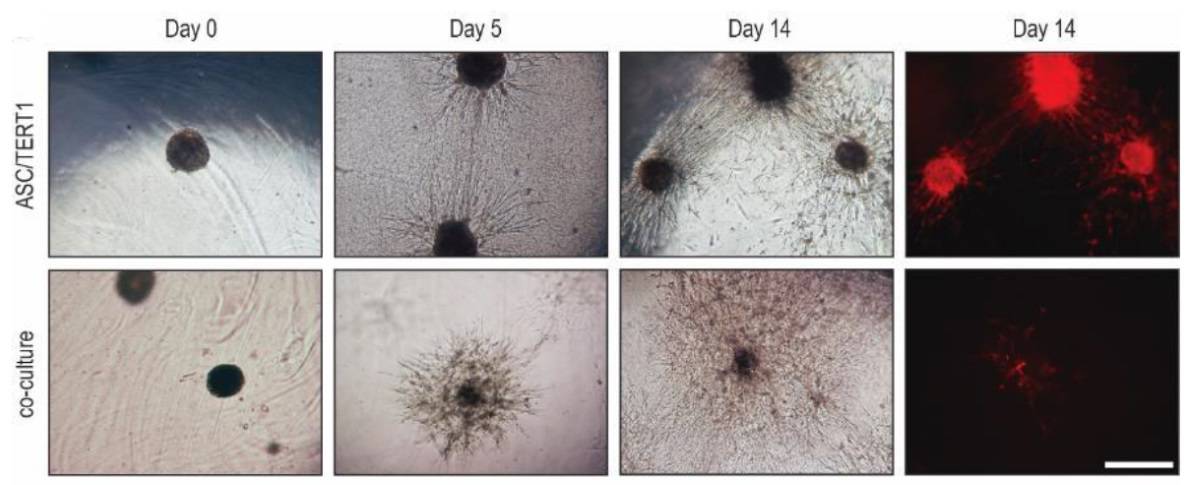
This strong outgrowth and matrix deposition suggests that the spheroids would likely also grow if delivered in vivo and thus could be used for cartilage repair. “This is one of the intended applications,” Nürnberger tells Physics World. “Spheroids could be implanted directly into the defect or be used as building blocks for bio-printing prior to implantation.”
The team is now investigating strategies to use the spheroids for cartilage defect regeneration, as well as in drug screening, since spheroid formation speed is altered by influences such as cytokines and drugs. “However, we are always thinking of new possibilities to apply our spheroid formation approach to new applications,” adds Nürnberger.



