Spinal surgeries, particularly those involving the spinal cord and nerves, carry an inherent risk of neurological injury. For such procedures, surgeons use intraoperative neuromonitoring (IONM) to map electrical activity in the spinal cord and lower the risk of damage during this delicate procedure.
IONM is currently performed using electrodes on the scalp and limb nerves to record responses on one to electrical stimulus of the other, to ensure that the critical neuronal connections between the two in the spinal cord remain intact during surgery. But this indirect approach requires high-amplitude stimulation, provides limited spatiotemporal information to guide the surgical procedure, and can lead to false positives and negatives. And while IONM can detect inadvertent neurological injuries after they occur, the large number of measurements required to produce a useful signal prevents its use for real-time monitoring.
Researchers in Shadi Dayeh’s Integrated Electronics and Biointerfaces Laboratory, along with neurological surgery colleagues led by Joseph Ciacci at UC San Diego and Ahmed Raslan at Oregon Health & Science University, have come up with a way to overcome these limitations. They propose that direct recording of somatosensory evoked potentials (SSEPs) from the surface of the spinal cord should increase the quality and resolution of the recordings, reduce the data recording time and enable high-definition spatiotemporal mapping.
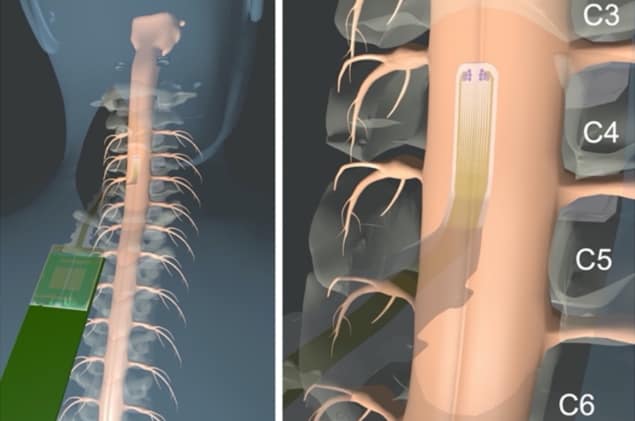
To test this hypothesis, the researchers developed a microelectrode array with hundreds of channels that can be placed on the exposed spinal cord during surgery, describing their design in Science Translational Medicine.
The team created the microelectrode arrays using low-impedance contact materials, with the electrodes fabricated on 6.6-µm parylene C substrates. These arrays are much thinner than current (1 mm thick) clinical spinal cord grids and thus conform better to the spinal surface. In addition, the arrays are transparent, giving the surgeon an unobstructed view of the surface spinal anatomy with the grid in place.
Clinical assessment
First authors Samantha Russman, Daniel Cleary and colleagues tested their arrays in six patients undergoing tumour or biopsy surgery in the spinal cord. In the final design of the microelectrode arrays (used for patients 4 to 6), the array comprised 372 platinum nanorod contacts of 30 µm diameter, arranged in a 12 × 31 rectangular array to cover an area of 3.85 x 12 mm. The arrays were temporarily implanted directly on the participant’s spinal cord during surgery.
The researchers delivered electrical stimulation to both upper and lower limb nerves. They initially used stimulation currents at clinical amplitudes of between 30 and 50 mA, then decreased the current to half, one-quarter and one-eighth of these values, with the lowest stimulation amplitude of 4 mA (for participant 5). During surgery, the team captured SSEP patterns from the spinal cord surface, performing epidural and subdural recordings before resection and subdural recordings afterwards.
The microelectrode array recorded dynamic patterns of SSEPs with high sensitivity, outperforming a clinical IONM system and requiring shorter data recordings. The array could record both epidural and subdural responses at stimulation currents well below those used clinically and could resolve postoperative evoked potentials when standard IONM could not.
One major task during spinal cord surgery is identification of the midline – the only safe corridor to the inner structure of the spinal cord. Unfortunately, there is no consistent anatomic landmark on the surface to the midline, which is often also distorted by inflammation, tumours or scarring.
Applying the high-channel-count array to the exposed spinal cord during surgery enabled the researchers to construct high-resolution 2D maps of spine activity. They used the spatiotemporal features of these recorded SSEPs to map the electrophysiological midline of the spinal cord with submillimetre precision.
The subdural recording identified the midline with better precision than epidural measurement, in agreement with the more localized response profiles seen for subdural placement of the microelectrode grid. This set-up could isolate ipsilateral responses to stimulation and outline a midline spanning two to five channels (700 µm to 1.75 mm) – a higher resolution than reported previously. The researchers note that this midline boundary was identified at clinical stimulation currents and persisted even when scalp SSEPs could not be identified.
Engineered spinal cord implants restore movement to paralysed mice
To validate whether smaller contacts can record high-quality SSEPs, the team fabricated an additional 54 contacts of variable diameter (30 to 480 µm) on the tip of the microelectrode array. The data showed that the 30-µm-diameter platinum nanorod contacts were as effective at recording as the larger diameters, validating their use for high-definition mapping from the surface of the spinal cord.
The researchers conclude that their microelectrode array shows promise to improve neurosurgical procedures and capture previously unexplored spinal cord activity. As these direct recordings do not require substantial trial averaging, once real-time data analysis and plotting are incorporated, the technology could provide a practically instantaneous IONM method. And in future, the team speculates, the system could offer additional applications such as monitoring, or even treatment of, spinal cord injuries.




