
Neurovascular cell interactions within the brain are critical to controlling cerebral blood flow and regulating which compounds and proteins can cross the blood-brain barrier (BBB). When these neural and vascular cells run into damage or dysfunction it can lead to the cognitive decline observed in vascular dementia. Research groups have strived to replicate neurovascular interactions in vitro to analyse the underlying mechanisms of dementia. Progress of such research, however, has been hindered by difficulties in modelling the interaction between different cell types or ensuring that the correct nutrients reach the appropriate cell types.
Researchers from Seoul National University in South Korea have developed a microfluidic neurovascular-unit-on-a-chip that enables both an interactive interface between neural and vascular cell compartments, whilst also separating key nutrients to the appropriate cell compartments. The developed model enabled BBB formation in the central portion of the chip, with vascular and neural interactions being enabled by the specific chip dimensions (Scientific Reports 7 8083).
Noo Li Jeon and team produced the microfluidic device using photolithography and soft lithography techniques. This allowed for remarkable definition and resolution, which enabled the separation of fluids for neurovascular interaction. The model was produced by creating a negative “master” mould from plastic (SU-8), from which multiple silicon wafers (polydimethylsiloxane (PDMS)) with the specific detailing required were produced. The researchers encapsulated the cells within a fibrin hydrogel before seeding them on the chip. This provided a 3D architecture for cells, which is much more physiologically relevant than a planar 2D material. This hydrogel allowed cells to bind to and migrate through the 3D porous network of the hydrogel and enabled the flow of nutrients and growth factors to all regions of the chip.
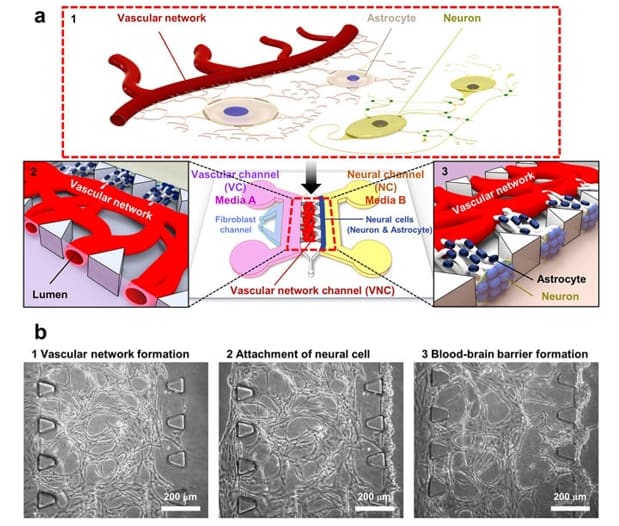
The researchers showed that injecting neural and vascular media into their respective components promoted cell growth and interaction compared with that seen when the cell media were mixed together. The separation of media compartments also displayed a positive effect on synaptic connections between neurons, in the form of increased expression of a synapse-associated protein. This established an improved synaptic development in neurons cultured with neuron-specific medium in the neural compartment.
The authors have suggested that this microfluidic BBB model can be used to screen drugs for BBB permeability in neurodegenerative diseases, whilst also reducing the number of animals used for research. By using cells from vascular diseases like vascular dementia, the model could be employed to dissect the specific cell-cell interactions that affect pathology. The authors also state that the model is incomplete as far as a full “neurovascular unit” is concerned, as it can only model the interaction between endothelial cells and astrocytes. Further introduction of the missing cell types within this interactive platform would provide a useful tool for more effective BBB and neurovascular research.



