The efficiency of perovskite LEDs (PeLEDs) is limited by electron traps that form due to structural defects. While defect passivation – the science of neutralizing these traps often with molecules – has improved PeLED efficiency, exactly how this works remains unclear. But now, work led by researchers at Linköping University reveals that hydrogen bonding plays a critical role in passivation. Their findings have huge implications for the commercialization of perovskite LEDs and solar cells.
Defect management
In the perovskite FAPbI3, defects are formed at the grain boundaries, which terminate the continuous arrangement of Pb2+, I– and CH(NH2)2+ (FA+) ions. Specifically, Pb2+ ions that are coordinated to six I– ions in the bulk of the crystal, end up unsaturated at the surface. This is bad news for PeLEDs as the resulting positive charge works to ‘trap’ electrons before they can release a photon and produce light.
Scientists have tried to alleviate this effect by treating the perovskite with electron-donating passivating agents (PAs), to bond to the unsaturated Pb2+ and neutralize the positive charge. It follows from this basic model that PAs with a strongly electron-donating character should offer the best passivation capability and lead to the most efficient devices.
It was therefore to the surprise of Weidong Xu and Feng Gao of Linköping University that they observed quite the opposite behaviour when comparing two PAs: HMDA and EDEA. These molecules are identical save for the two oxygen atoms in EDEA, which work to move electron density away from the passivating amino (-NH2) groups.
Based on popular understanding in the field, one would expect HMDA to coordinate more strongly to Pb2+. Nevertheless, it was the EDEA-passivated perovskite which performed best, with an external quantum efficiency (EQE) of 18% compared with just 11% when HMDA was used.
Light bulb moment
So what causes this unexpected behaviour? “After endless hypothesis and experiments we finally arrived at a possible explanation,” says Xu with relish, “Hydrogen bonding.” He proposes that the ability of the PA to bind with Pb2+ at the perovskite surface is just half the story. What’s missing is that if the electron-donating ability of the PA becomes too high, it is more likely to interact with neighbouring FA+ ions via hydrogen bonds, leaving the Pb2+ traps unpassivated. The researchers had unravelled the key to effective passivation of PeLEDs.
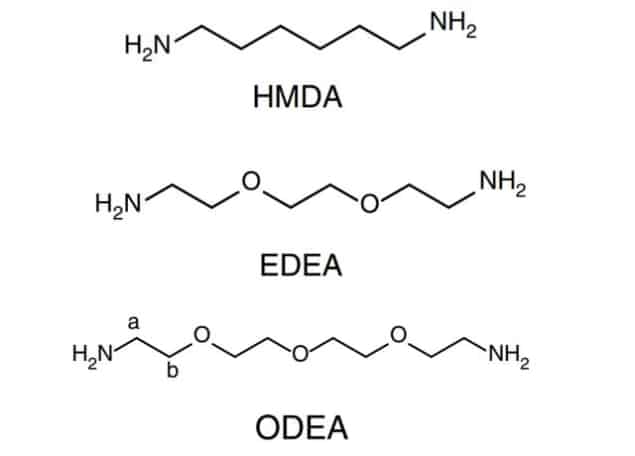
“Based on this new understanding, we set about designing new molecules to further boost the LED performance” explains Xu. To do this they made use of the inductive effect, adding extra alkyl groups (-CH3) or O atoms to increase or decrease the electron density at the passivating amino group.
After playing with the PA composition in this way the group landed on ODEA, a molecule that gave them a record PeLED EQE of 21.6%; a giant step forward given the previous record was just 14%. This impressive performance adds to the commercial viability of PeLEDs, which already exhibit lower fabrication costs and finer colour quality than OLEDs.
Perovskite homojunction reduces charge carrier recombination
But Xu asserts that there is more work to be done before we see PeLEDs on our screens, “The next step for us is to apply similar principles to improve the efficiency of perovskites that emit in the visible region.” Certainly, with the record EQE of blue-emitting PeLEDs at just 6.7%, their passivation technique could be central to the development of next-generation lighting and displays.
The impact of these PAs doesn’t just stop at PeLEDs either. “We believe the concept should work for improving the efficiency of perovskite solar cells, and our initial work on this looks promising,” says Xu. In any case, the key insights from this work should lead to a bright future for perovskite optoelectronics.
Further details can be found in Nature Photonics.




