Physicists in the US have shown how the electrical current flowing through certain molecular junctions can be switched on and off on by simply stretching or compressing the molecules. The discovery could be exploited to make switches in future molecular devices, and could also help in the understanding of resistance at the nanoscale.
In normal electronic devices, resistance is a well-understood property. When an electric field is applied across a metal, charge–carrying electrons begin to drift but bump into ions and impurities, which slow their motion. At nanometre distances, however, things are not so simple: electrons are able to “tunnel” across small insulating barriers with a finite probability. This means that the electrons can overcome obstacles without losing energy, and therefore without electrical resistance.
Tunnelling is an important consideration in designing nanoscale devices, but measuring its effect is not easy. In 2007, a team led by Latha Venkataraman at Columbia University in New York and Jeffrey Neaton of Lawrence Berkeley National Laboratory (LBNL) in California, managed to measure the conductance of amine-based molecules that were in contact with gold and successfully compare with theory. They touched the gold tip of a scanning tunnelling microscope (STM) onto a gold surface containing amine molecules and then retracted it, so that the tip linked to the surface only by a single, fragile molecular strand. When this strand broke other molecules hopped in to fill the gap, and from this the researchers could calculate the sudden jump in conductance.
Molecular bridge
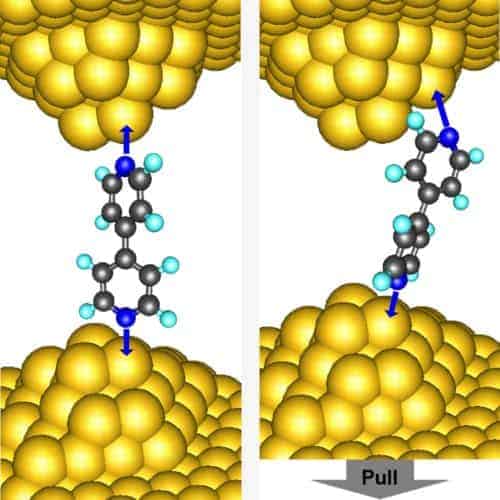
Now, the researchers at Columbia’s NSF Center for Electron Transport in Molecular Nanostructures, and LBNL’s Molecular Foundry have gone a step further and looked at different conductance states in the molecular strand bridging a STM to a gold surface. Rather than use amines they have used bipyridine, a molecule with twin benzene-like rings that contain nitrogen. They found that bipyridine has two resistance states: when it is bonded to the STM at an angle the resistance is low and the junction is “on”; when it is bonded vertically the resistance is high and the junction is “off”. In fact, the researchers found that they could switch the junction between the on and off resistance states simply by pushing and pulling the STM tip (Nature Nanotechnology doi:10.1038/nnano.2009.10).
Venkataraman, Neaton, and colleagues believe the different resistance states result from electron tunnelling through the conductive “pi” orbitals of the bipyridine molecule. The different orientations of the molecule change the amount of overlap between the quantum wavefunctions of the pi orbitals and the incident electrons from the gold electrodes. When bipyridine is at an angle the overlap is greater, which increases the chance of tunnelling and therefore decreases the resistance.
A better theory
“One outcome of this study is an improved ‘first principles’ theoretical approach, free of empirical parameters, for conductance in certain classes of nanoscale molecular junctions,” Neaton told physicsworld.com. “Working with experiments, we will continue to develop and extend the range of our theories to junctions and phenomena involving more complex molecules.”
Neaton explains that his team are particularly interested in looking at certain asymmetric molecules with the ability to separate charge, which are used in some organic photovoltaic materials. “Extended studies of such molecular junctions may shed further light on how we might enhance the efficiency of charge separation and transport — and thereby light harvesting — in organic and hybrid organic–inorganic nanomaterials,” he adds.