Researchers from Austria have developed a non-invasive method for assessing hypoxia and neovascularization in breast tumours using MRI data. Their novel MRI approach, which offers the potential to improve tumour characterization and facilitate tailored treatment, is designed for easy integration into a diagnostic MRI protocol.
Tumour hypoxia is a condition in which tumour cells have low oxygen concentrations compared with healthy tissues, resulting from an imbalance between oxygen supply and consumption. Its presence in a breast tumour increases the cancer’s resistance to radiotherapy and can decrease the effectiveness of some chemotherapy treatments. Tumour hypoxia is a strong prognostic factor for disease progression, development of metastases, and the overall survival of a patient.
Methods to accurately assess tumour hypoxia, neovascularization and breast cancer heterogeneity, however, have been limited. Additionally, the levels of oxygenation within a tumour are highly variable from one region to another and can change over time. Existing imaging approaches include PET, which has high costs, or near-infrared spectroscopy, which is of limited value due to its low spatial resolution.
Two recently developed MRI techniques offer a quantitative measurement of hypoxia and have been pioneered in patients with brain cancers. Advanced quantitative blood oxygenation level dependent (qBOLD) imaging can quantify the tissue oxygen tension and provide a direct measurement of tumour hypoxia. Meanwhile, vascular architectural mapping (VAM) can measure and quantify microvascular vessel diameter and architecture.
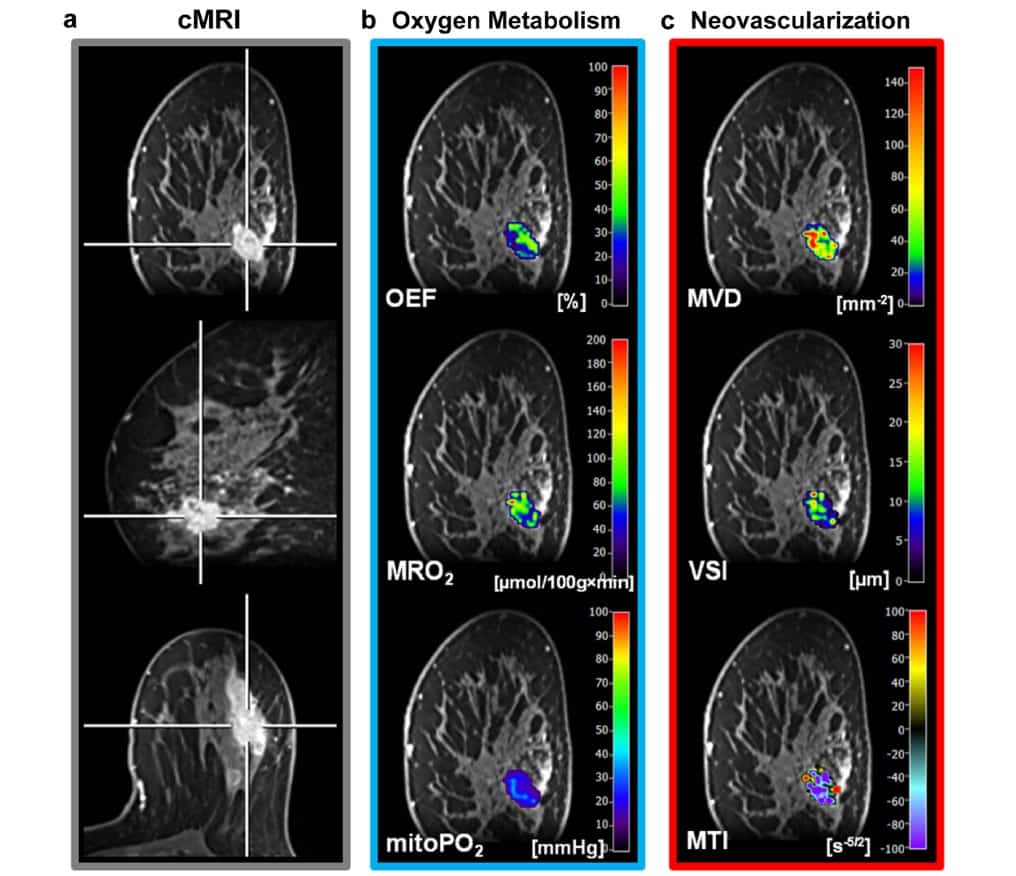
Radiologist Katja Pinker and colleagues at the Medical University of Vienna hypothesized that this MRI-based assessment of hypoxia and neovascularization should be feasible in breast tumours. To validate their technique, the researchers performed multiparametric breast MRI, including qBOLD imaging and VAM, on 20 patients with benign and malignant breast tumours. They used the acquired data to create MRI biomarker maps of oxygen metabolism and neovascularization (Mol. Imaging Biol. 10.1007/s11307-018-1298-4).
All patients had a suspicious lesion with a diameter of at least 10 mm identified on mammography or breast ultrasound. Thirteen patients were diagnosed with invasive ductal carcinoma and seven had benign tumours.
For the MRI sequence protocol, the team first acquired diagnostic MRI sequences, followed by T2- and T2*-mapping sequences for qBOLD imaging, coronal diffusion-weighted imaging (DWI) for VAM, and the native T1w FLASH sequence for dynamic contrast enhancement (DCE) perfusion. The authors note that only a single dose of gadolinium-based contrast media, injected during the dynamic susceptibility contrast (DSC) gradient echo spin echo (GESE) perfusion sequence, was needed.
The researchers used custom-made MatLab software to post-process qBOLD and VAM data and calculate quantitative MRI biomarker maps of oxygen extraction fraction, metabolic rate of oxygen and mitochondrial oxygen tension. They used these maps to measure tissue hypoxia and neovascularization, including microvessel radius, density and type of vascular architecture.
The resulting MRI biomarker maps demonstrated intra-tumoural spatial heterogeneity with a broad range of biomarker values. The researchers determined that malignant lesions showed significantly higher microvessel density and metabolic rate of oxygen, lower mitochondrial oxygen tension and lower (more pathologic) microvessel type indicator.
“This indicates that breast cancer consumes more oxygen and is more hypoxic and neovascularized than benign tumours,” they authors write. “These findings confirm the importance of tumour hypoxia and neovascularization as powerful physiological stimuli that can be exploited as a tumour-specific condition and can be used to design hypoxia-based imaging biomarkers and hypoxia-activated anti-cancer drugs.”



