A new magnetic resonance imaging (MRI) technique maps the location of cells labelled with light-emitting molecules even when they are located deep within organs and other tissues. The technique, which works by detecting changes in blood vessels triggered by the presence of bioluminescent proteins, overcomes a major limitation of optical imaging. It could find use in biomedical applications such as probing tumour growth, measuring changes in gene expression and studying brain cell function.
Biologists often use light-emitting proteins to label cells, as it enables them to follow processes such as cell signalling, metabolism and many other cellular functions by tracking where these proteins go. However, while these bioluminescent proteins work well as indicators within cells, they are not as good for imaging structures deep in tissues and organs because these objects absorb and scatter visible light too much.
Locating the source of light emission
Biological engineer Alan Jasanoff and colleagues of the Department of Biological Engineering at the Massachusetts Institute of Technology (MIT) in the US have now developed a new way to detect bioluminescence. Their method begins with genetically engineering blood vessels to carry a photosensitive protein – in this case, an enzyme known as Beggiatoa photoactivated adenylate cyclase (bPAC).
When the engineered blood vessels are illuminated with light, the protein within them makes them dilate. This has the knock-on effect of altering the balance of oxygenated and deoxygenated haemoglobin within the vessels. Because these forms of haemoglobin have different magnetic properties, the shift between them can be detected using MRI. This enables the researchers to locate where light emissions are happening with high precision.
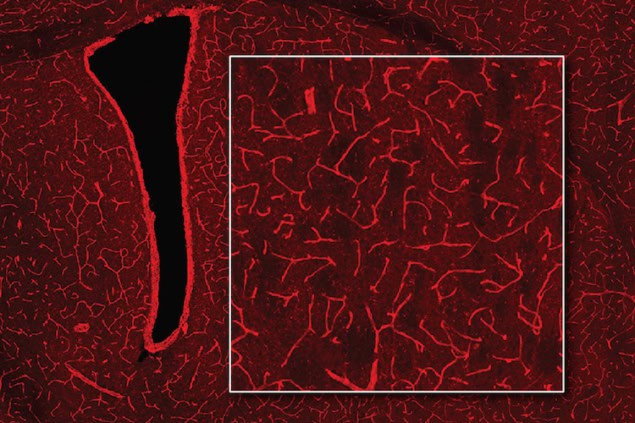
Jasanoff and colleagues tested their technique on the blood vessels in rat brains. “Blood vessels form a network in the brain that is extremely dense. Every cell in the brain is within a couple dozen microns of a blood vessel,” Jasanoff explains. “Our technique, which we have dubbed bioluminescence imaging using haemodynamics, or BLUsH, works by essentially turning the vasculature of the brain itself into a three-dimensional camera.”
Blood vessels become light amplifiers
Each blood vessel is like a pixel, he adds, responding to nearby sources of light in the tissue. “Since vascular changes are readily detectable by noninvasive readouts like MRI, BLUsH enables us to perform optical imaging through tissue that wouldn’t normally be easily accessible with optical techniques.”
The most difficult part of getting the technique to work, he tells Physics World, is getting the genetic modification of blood vessels properly targeted. “We are working on simplifying this,” he says.
High-resolution MRI enables direct imaging of neuronal activity
By making luminescent proteins detectable via MRI or other noninvasive imaging techniques, BLUsH could help scientists study how cellular level processes lead to emergent phenomena such as brain-wide activity dynamics. It could also be useful for discovery-oriented science and clinical research in animal models. For example, the researchers suggest that studies of how gene expression changes during embryonic development and cell differentiation, or when new memories form, might benefit. Luminescent proteins could even help map anatomical connections between cells, revealing how cells communicate with each other.
For its part, the MIT team hopes to use BLUsH to study brain plasticity — the process that underlies learning and memory. “We also want to use this technique to read out measures of neural signalling,” Jasanoff says.
The work is detailed in Nature Biomedical Engineering.