Biohybrid microrobots, which combine the motility of natural micro-organisms with the multifunctionality of synthetic components, are being studied as an alternative to purely synthetic microrobots. Designs based on biocompatible and deformable materials serve as novel platforms for use in vivo, enhancing the potential of microrobots for biomedical applications. In a recent study reported in Nature Materials, researchers describe a bioinspired microrobot platform consisting of nanoparticle-modified algae for active delivery of antibiotics to treat lung disease.
Nanoengineers at the UC San Diego Jacobs School of Engineering modified microalgae, a natural organism, by covering its surface with drug-loaded polymer nanoparticles (NPs) coated with the membranes of neutrophils (a type of white blood cell). The researchers named their new design the “algae-NP-robot”.
The work is a joint effort between the labs of Joseph Wang, an expert in micro- and nanorobotics research, and Liangfang Zhang, whose expertise lies in developing cell-mimicking nanoparticles for treating infections and other diseases. The researchers chose to first test the algae-NP-robot for in vivo antibiotic delivery to treat bacterial lung infections.
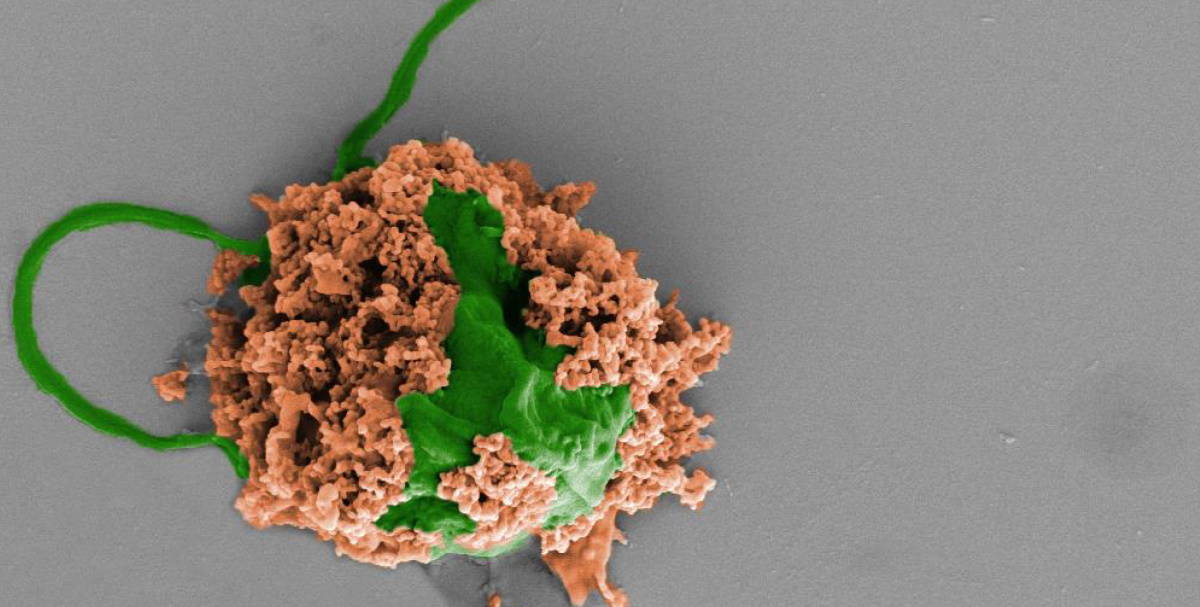
The researchers modified the algae by using click chemistry (which won the 2022 Nobel Prize for Chemistry) to couple the algal surface with antibiotic-loaded polymer NPs. Next, they administered the algae-NP-robot directly into the lungs of mice with bacterial pneumonia, via a tube inserted in the trachea.
The algae provide swimming motion in the lungs, allowing the microrobots to move around and deliver antibiotics directly to bacteria in the animals’ lungs. The algae-NP-robots safely eliminated pneumonia-causing bacteria, with all treated mice surviving past 30 days. In contrast, untreated mice died within three days. The team noted that treatment with microrobots was more effective than injection of antibiotics into the bloodstream.
The presence of neutrophils on the microrobot surface helps neutralize inflammatory molecules produced by the bacteria in the lungs of the mice, as well as by the animal’s immune system. This method of delivery, using live algae microrobots, effectively inhibits phagocytosis by macrophages (another type of white blood cell) and prolongs the retention of algae-NP-robots inside the infected lungs. This is a significant achievement since macrophages like to engulf and digest any foreign substances inside the immune system.
To gain further insight into the clearance mechanism, the researchers studied the motion and cargo-carrying behaviour of the algae-NP-robots in simulated lung fluid. The simulation study combined with in vivo drug delivery highlights the platform’s potential to safely provide therapeutic efficacy with drug-loaded algae-NP-robots.
Bacteria-based microrobots show potential for cancer drug delivery
“With an IV injection, sometimes only a very small fraction of antibiotics will get into the lungs. That is why many current antibiotic treatments for pneumonia do not work as well as needed, leading to very high mortality rates in the sickest patients,’’ says co-author Victor Nizet.
This research is still at the proof-of-concept stage. Future steps involve understanding the mechanisms underlying the interaction of microrobots with the immune system. However, Zhang believes that the new design will push the boundaries in the field of targeted drug delivery.