“About 785 million people are living without access to safe and clean drinking water; 140 million people in more than 50 countries have been exposed to arsenic-contaminated water.” These were the stark opening statements from Muhammad Abbas, speaking at the recent APS March meeting.
Arsenic poisoning is one of the most significant public health concerns worldwide. Arsenic is used in semiconductors, pharmaceuticals, wood preservatives, insecticides and chicken feed, and it leaches into the groundwater. Long-term exposure can lead to cancers of the kidney, liver, lungs and skin, as well as causing skin diseases and other health issues such as hypertensive heart disease. As such, the World Health Organization and Environmental Protection Agency recommend a maximum limit of 10 µg/l arsenic in drinking water.
Despite these serious health concerns, testing for arsenic in water currently requires expensive laboratory instruments that cannot be used for on-site detection and are unsuitable for developing nations. Abbas and colleagues at LUMS in Pakistan hope to address this shortfall by designing a low-cost sensor that can detect arsenic in drinking water. “We aim to develop a sensor that’s sensitive and selective, robust and reliable, affordable, portable and easy to use for local technicians,” he said.
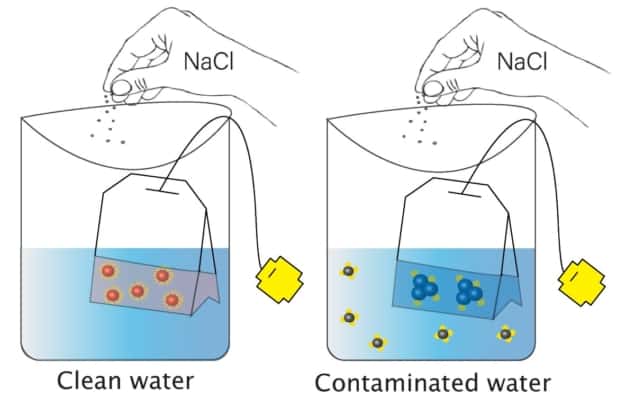
The sensor will be based on gold nanoparticles (AuNPs), which are excellent candidates for sensing applications as they absorb in the visible spectrum and change colour according to their size, shape and surface chemistry. To create their sensor, Abbas and colleagues coated AuNPs with dihydrolipoic acid. This coating stabilizes the nanoparticles, which form in a dispersed state in water and are wine-red in colour. Adding an electrolyte such as salt does not affect the AuNPs, which remain dispersed and stay red.
If the water contains arsenic, however, the arsenic will bind to the dihydrolipoic acid, making it unavailable to protect the AuNPs surfaces. In this scenario, adding salt causes the AuNPs to aggregate and change colour. “This aggregation is directly proportional to the amount of arsenic present, which decides the strength in the colour change,” explained Abbas, now a PhD student at the University of Texas at Dallas.

To test this approach, the team performed UV-visible spectroscopy on AuNP solutions containing different concentrations of arsenic. “We could see a visual colour change with increased amounts of arsenic, with the nanoparticles changing from red towards blue,” said Abbas. Scanning electron microscopy images of the AuNPs before and after addition of arsenic confirmed the clumping mechanism that caused the colour change.
The sensor’s detection limit was 50 µg/l (50 parts per billion) of arsenic when viewed with the naked eye, or 3 µg/l using UV-visible spectroscopy. This sensitivity is lower than that offered by existing high-tech methods, such as atomic fluorescence spectroscopy, atomic absorption spectroscopy or mass spectrometry, which can detect up to parts per trillion of arsenic. But Abbas emphasizes that these systems are costly, not portable and need trained personnel to operate them.
“The colourimetric method is affordable and portable. The limit of detection is lower right now, but it can be improved,” he said.
The team also investigated potential interference from a range of other metal contaminants and found that, with the exception of mercury, none of the metals interfered strongly, and even mercury only impacted the absorption spectrum slightly.
“This suggests that the sensor would be selective and not disturbed by other elements present in the drinking water,” Abbas explained. “In future, this method may lead to the design of a microfluidic device for detection of arsenic.”



