Recent work has suggested that prostate tumours with high nerve densities are more likely to grow and spread than those with low nerve densities. Now, a team in China and the US has shown that such high-risk cases can be identified using a combination of MRI, magnetic particle imaging (MPI) and functionalized iron-oxide nanoparticles. In experiments with mice, the researchers also used the same nanoparticles to deliver a drug that blocks nerve function, slowing the spread of prostate cancer and improving the animals’ survival rate (Science Advances 10.1126/sciadv.aax6040).
Tumour development and proliferation is a complex process involving multiple tissue types and structures. Angiogenesis – the formation of blood-vessel networks – has long been recognized as a vital component, prompting the formulation of antiangiogenic drugs intended to control tumour growth. Only in the last decade, with studies of prostate cancer progression specifically, has the nervous system emerged as a similarly important part of the process.
Wenting Shang of the Chinese Academy of Sciences (CAS) – who led the research with Huijuan You of CAS and Huazhong University of Science and Technology – likens the development of cancer to the construction of a new building. “When a new building is built, you need to set up water pipes and wires for water to flow smoothly and the lights to blaze,” says Shang. “Angiogenesis can be seen as cancer cells building water pipes; a dense network of nerves can be seen as cancer cells laying wires.”
Spotting tumours that have laid down such a network of nerves could help determine how dangerous a given case of prostate cancer is likely to be. This would let clinicians tailor the scale of the intervention to suit the risk, avoiding overtreatment of less aggressive tumours.
Unfortunately, nerve density is a difficult tissue property to measure using typical imaging techniques. For example, while MRI – the method of choice for prostate-cancer imaging – can usually delineate a tumour clearly enough, those that are dense with nerves look very similar to those with undeveloped nervous systems.
To solve this problem, Shang, You and colleagues developed a contrast agent that targets nervous tissue specifically. The team started with nanoparticles of iron oxide, which have already found use in both MRI and MPI, and joined them to the nerve-binding peptide NP41.
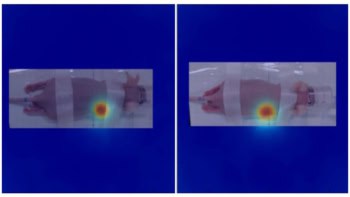
Injected into the bloodstream of a mouse, the contrast agent disperses through the animal’s entire circulatory system but is quickly metabolized and removed. Because of the enhanced permeability and retention effect in the tumour, however, the nanoparticles accumulate in the cancerous tissue, where they bind preferentially to proteins around the nerve fibres. The researchers looked for this effect in mice with prostate tumours that had either been allowed to grow without interference (yielding high nerve densities) or from which the nerves had been surgically or chemically compromised (yielding low nerve densities).
Twenty-four hours after injection of the contrast agent, tumours with high nerve density showed up clearly on MRI scans – and even more so using MPI. In low-nerve-density tumours, in contrast, the nanoparticles were virtually undetectable with MRI but produced a faint signal using MPI. The researchers think that MPI therefore offers an ideal method to visualize nerve density in prostate tumours.
Given the importance of dense nerve networks for tumour growth and proliferation, the team proposed that interventions that not only highlight but also target the nerves might reduce a cancer’s aggressiveness. To test this, they added another component to the functionalized nanoparticles – a beta-blocker called propranolol, which affects nerve function.
Mice injected with the propranolol-conjugated nanoparticles tended to survive for longer: 45 days after the treatment, 83% of the propranolol-treated group were still alive, compared with 40–50% of those given either propranolol-free nanoparticles or propranolol alone.
The treatment also seems to have been without significant side effects. Despite the nanoparticles entering each mouse’s general circulatory system, they tend to deliver their propranolol cargo only in the low-pH microenvironment of the tumour. Combined with the preferential accumulation of nanoparticles in the tumour, this means that the mouse’s wider nerve network is relatively unaffected.
The researchers think that the procedure could produce similar results in solid tumours elsewhere in the body, as the relationship between cancer propagation and nerve networks is probably not prostate-specific. There is still much more work to be done before it is ready for the clinic, however.
“Firstly, MPI technology is still in the pre-clinical stage,” says Shang. “Secondly, our probes are not yet mature for clinical use, and still need to be optimized. We plan to conduct more comprehensive toxicological studies of our probes in the next stage.”