Neuroblastoma is one of the most common cancers in children and requires surgical removal. During this surgery, there is an increased risk of damage to healthy tissue. Real-time imaging techniques are therefore required to discriminate between neuroblastoma lesions and non-cancerous tissue. However, current techniques do not allow real-time imaging, since the blood–brain barrier hinders the delivery of imaging agents.
With this in mind, researchers from the CAS Key Laboratory of Molecular Imaging, Institute of Automation, Chinese Academy of Sciences have developed gadolinium oxide nanoplates conjugated with rabies virus glycoprotein (RVG) peptide that can target neuroblastoma cells. The project aims to circumvent the limitations of current imaging techniques and assist in both the detection and surgical excision of neuroblastoma tumours (Acta Biomater. 10.1016/j.actbio.2019.01.042).
Nanoplates cross the blood–brain barrier
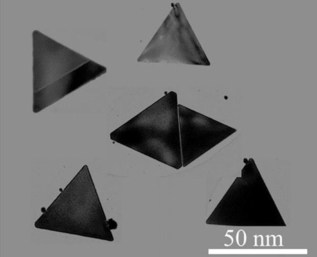
The nano-structures contain three main elements, each one with its own function. First, the triangular nanoplates act as a carrier and are small enough to penetrate the brain capillaries. The modified RVG peptide then enables infiltration through the blood–brain barrier and promotes uptake by neuroblastoma cells. Lastly, the nanoplates also include a fluorogenic dye, which allows their detection using real-time fluorescent imaging. Once fabricated, the researchers also demonstrated that the nanoplates were not cytotoxic and were stable over 30 days, making their storage possible.
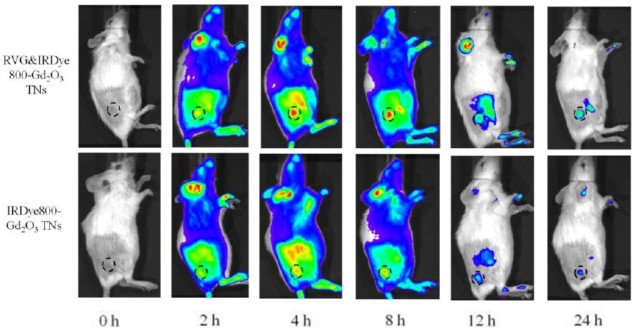
To demonstrate the potential of these nanoplates, the researchers injected them in mice with induced neuroblastomas in the brain and under the skin. They then imaged the animals with both MRI — which detects the gadolinium oxide — and fluorescence imaging — which detects the fluorogenic dye, using nanoplates without RVG peptide as a control. The nanoplates with the RVG peptide attached produced two to three-fold higher intensity MRI and fluorescence signals in the tumours, proving the ability to precisely detect the tumour.
Taking it a step further, mice with brain neuroblastoma underwent surgery and the researchers used the nanoplates to image the brain with fluorescence imaging in real time. The system provided accurate tumour detection and guidance for excising the tumours, which increased the post-surgical survival of the animals from 0% to 80% after 40 days. The researchers further proved the precise tumour detection when analysing the brains with histology, where the tumour was accurately depicted.
A potential clinical system
The authors believe that these nanoplates could be used in humans to guide neuroblastoma excision surgery, improving the outcomes by reducing the damage to healthy tissue. This potential application is further supported by the nanoplates’ low cytotoxicity and targeting accuracy, meaning that this system could significantly improve diagnosis and treatment for neuroblastoma patients.



