The environment has been a hot topic throughout the history of nanotechnology, yet despite decades of research, definitive conclusions on the environmental impact of nanoparticles remain elusive. Anna Demming reports on the latest efforts to assess them

For early nanotechnologists, debates about the field’s environmental effects centred on a scenario as frightening as it was unlikely. Despite having more in common with science fiction than science, the idea – first proposed in 1986 – that zillions of self-replicating nanobots might somehow consume all biological matter on Earth, leaving only a useless “grey goo” behind, went on to colour debates about the field for years to come.
These days, the “grey goo” terror has long since subsided. As nanomaterials migrated from the annals of science fiction into real-world applications, evidence of their positive contributions grew, and this has helped to shape a more measured view of their potential hazards to the environment or human health. As a result, says Boris Lau, an environmental engineer at the University of Massachusetts at Amherst, US, “substantial progress has been made to shift the perspective from ‘nano is dangerous’ to ‘nano can be made safe’ ”.
Despite this progress, however, questions about the hazards of nanomaterials have not gone away. Even within Lau’s statement, “can” seems to be the operative word. In practice, the job of establishing protocols for the safe use of nanomaterials is far from complete. One reason for this is that the same characteristics that give nanomaterials their unique advantages also make it hard to assess their risks. For instance, particles with nanoscale dimensions can access places that might otherwise be difficult if not impossible to reach – something with great advantages for applications such as device monitoring and medicine. Because of their small size, nanoparticles also have a particularly high surface area per unit volume, often with a high proportion of edges, which can help catalyse chemical reactions and enable high-sensitivity detectors. Finally, size-related effects produce unique electronic, optical, thermodynamic and mechanical properties that are not observed in bulk materials. These properties can be tuned for optimum performance in a given function through small adjustments in size, shape or composition.
As attractive as these attributes are, they also make for a legion of possibilities as to where nanomaterials may end up, and what impact they may have when they get there. Even a nanomaterial’s composition may be open to question. Because each batch typically contains variations on the theme of the intended product, different possible identities for individual particles start to proliferate from the moment they are produced. During a November 2014 NanoEHS workshop on the environmental health and safety of nanomaterials (2016 Env. Sci.: Nano 3 15), Lau and other participating researchers stated the problem neatly. In reporting on the outcomes of this workshop, the group observed that “simplifying complexity is no easy feat when the simple itself is complicated.”
Precision synthesis
Many research groups working on “green” nanotechnology are motivated by a sense of responsibility towards the planet. However, efforts to develop alternative synthesis methods that produce more consistent products, and do not rely heavily on toxic substances and extreme synthesis conditions, have also led to other benefits.
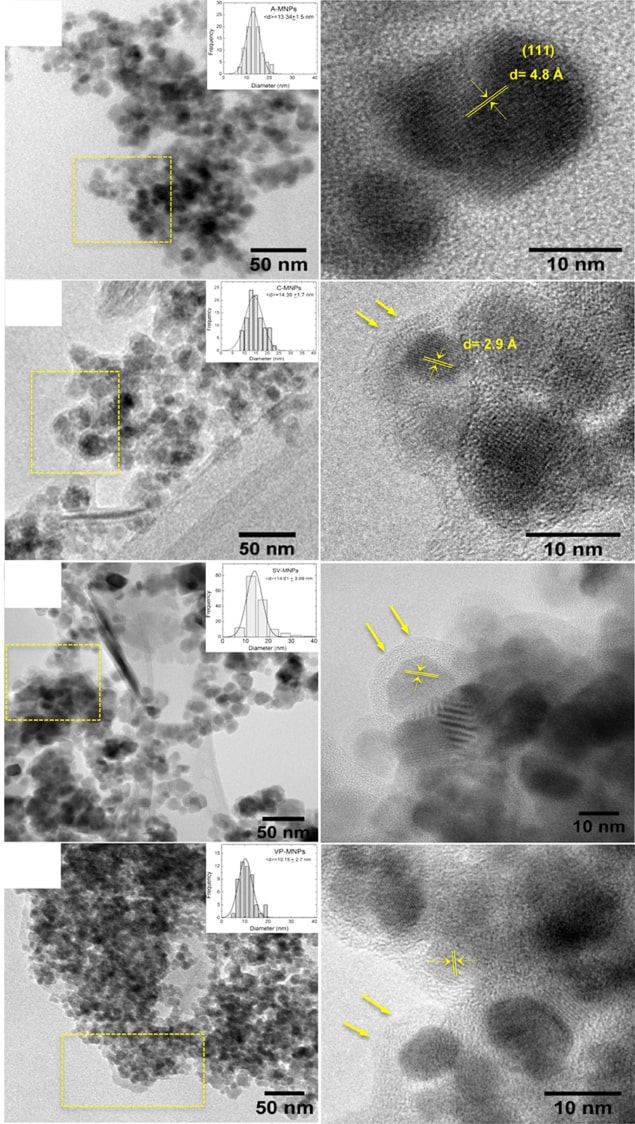
Take silver nanoparticles. Researchers have recognized their potential uses for decades, with proposed applications ranging from antimicrobial coatings to plasmonics and next-generation electronics. Over the years, the reduction of silver ions from solution became the preferred approach to synthesizing them, but this process often meant using toxic reductants and surfactants to cap the growth of precipitates at a particular size. In 2009, however, researchers at the Technical University of Wrocław, Poland, described an alternative strategy using fungi as the reducing agent. “Micro-organisms that are exposed to pollutants in the environment, such as metal ions, have a remarkable ability to fight that metal stress,” they reported. They focused their study on the filamentous fungi Penicillium – the same family of fungi from which the common antibacterial drug penicillin is derived. From experiments with the fungi extracted from soil and immersed in silver nitrate solution, the researchers concluded that silver ions are trapped on the fungus surface where they are reduced, slowly forming silver nanoparticles. The approach is environmentally friendly and easy to handle, and formed part of a growing movement towards biological synthesis.
Plant extracts are another “green” means of synthesizing nanomaterials at faster rates than is possible with fungi. In 2018 Jaime Santoyo-Salazar of Mexico’s Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional and colleagues used Cinnamomun verum and Vanilla planifolia (sources of the familiar cinnamon and vanilla flavours used in cooking) as both the reductants and capping agents to produce iron oxide nanoparticles that can be used in hyperthermia treatments for cancer (2018 Nanotechnology 29 074001). The researchers point out that as well as minimizing harm to the environment, their method avoids the need for protracted procedures to separate the product from any harmful chemicals used to produce it – a significant advantage for nanomaterials used in medicine.
As well as limiting a nanomaterial’s applications, contaminants from chemical synthesis can also compromise its properties. Graphene often comes up trumps in terms of impressive characteristics, and even before Andre Geim and Kostya Novoselov won the 2010 Nobel Prize for Physics for discovering its amazing properties, demand for industrial-scale approaches to graphene production was growing. In 2009 Xing-Hua Xia and colleagues at Nanjing University and Northwest University in China suggested reducing exfoliated graphene oxide at a graphite electrode by electrochemical means, which avoids the excessive reducing agents used in the photocatalytic reduction of graphite oxide (2009 ACS Nano 3 2653). As well as offering a fast and green approach (due to the absence of toxic solvents), Xia and colleagues also showed that the graphene they produced had fewer contaminants. Their method remains widely used.
Despite these improvements in nanomaterial synthesis, however, batch heterogeneities persist. “Nanomaterials that rely on reduction, nucleation and growth are sensitive to seemingly every kinetic variable (temperature, solvent, impurities, glassware etc),” observed Lau and his co-authors in the NanoEHS paper. “Hence, the observed physicochemical properties can arise from a single nanoparticle population rather than the average population.” For advocates of cleaner, greener nanotechnology, that presents a quandary. How do you safeguard against potential harm from materials if you can’t even be sure what they are?
Competitive obstacles
Part of the answer lies in better techniques for characterizing nanomaterials. Here, too, the field has come a long way. In 1990, when the first academic journal dedicated to the field (Nanotechnology – which, like Physics World, is published by IOP Publishing) was launched, even overcoming temporal variations in the shape of features was a major feat in studies at the nanoscale. The journal’s founding editor-in-chief, David Whitehouse, described these early studies as “akin to performing a surgical operation with a blunt instrument on an excited jelly”.
Since then, parallel advances in scanning-probe techniques based on atomic force, tunnelling currents and optical near-fields and electron beams; Raman, infrared and mass spectroscopy; and X-ray diffraction mean it is now possible to probe a nanomaterial’s vital stats (morphology, composition, electronic and mechanical properties, and so on) with atomic resolution and beyond, often in real time. These and a plethora of other techniques provide important quality assessments and can also aid efforts to audit engineered nanomaterials once they are in circulation.
Here again, though, “can” is the operative word. If a manufacturer finds that adding nanomaterials to its product gives it a competitive advantage, it is unlikely to advertise how that process works or provide meaningful details about the recipe. As Bernd Nowack, group leader of environmental risk assessment and management at Empa (the Swiss Federal Laboratories for Materials Science and Technology) pointed out during a plenary talk at Nanotech France in 2016, “One reason why quantitative data are difficult to obtain, or that even qualitative data are difficult to estimate, is because manufacturers do not necessarily report details or quantities of specific manufactured nanomaterials used in products.”
Cradle-to-grave sustainability
In addition to uncertainties about the intended or actual nanomaterials manufactured in industry, post-production processes can also cause the possible identities and trajectories of nanomaterials to multiply. As Nowack and fellow Empa researcher Denise Mitrano observed in a 2017 review (Nanotechnology 28 072001), the manufacturing and production portions of a product’s life cycle usually take place in a controlled, industrial setting. In contrast, they wrote, “The use and disposal of nanoproducts in the consumer realm is decidedly less predictable of specific MNMs [manufactured nanomaterials] from various products.”
In their review, Nowack and Mitrano highlighted the need for what they describe as a “life-cycle based aging paradigm” for nanomaterials, stressing the importance of real-world tests to identify realistic ways that particles can transform. “It is of course important to just look at how nanomaterials are behaving in the environment,” Mitrano says. “But if you actually look at how they are put into products or how they are released from products during their product life cycle, then you may be able to change some portion of their manufacturing or use, so that fewer nanomaterials are released or so that they may be released in a less harmful way.”
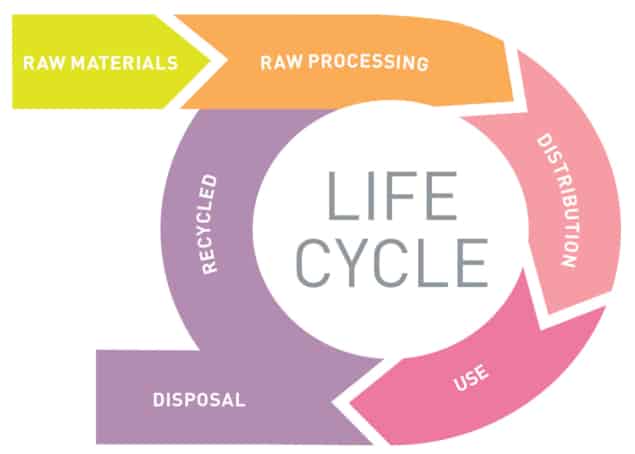
Mitrano, Nowack and fellow researchers involved in the EU’s Sustainable Nanotechnologies (SUN) project have developed cryomilling techniques and weathering tests to produce samples of “aged” nanomaterials for study. Mitrano’s work has focused on the use of silver nanoparticles in textiles, where particles may experience washing and UV exposure before ending up in landfill – a life cycle that can transform the original pristine nanomaterial in different ways than would occur if those same silver particles had been used in (for example) paint. Because conventional textile components such as dyes have long required safety assessments, Mitrano was able to use some of these standardized procedures to create readily comparable investigations. Developments in analytical techniques have also made it possible for her and her colleagues to perform measurements of nanoparticle concentrations at environmentally relevant levels of parts per trillion.
For silver nanoparticles in textiles, the conclusions seem encouraging. Mitrano has found that dissolved ions are often more toxic than the nanoparticles themselves, which demonstrates the benefits of transformations during the material’s life-cycle so that the nanoparticles are more resilient to dissolving. For example she points out that oxidation or sulphidation, as in the case of silver nanoparticles, can lead to more stable particles that are often much less toxic when they’re exposed. That is good news, but of course, the devil is in the detail, because even the impact of sulphidation can be life-cycle dependent.
Working with his students at Massachusetts–Amherst, Lau studied the impact of partial sulphidation of silver nanoparticles – a process that is highly prevalent when the nanoparticles are subjected to standard waste-water treatments. Previous studies had generally considered that, during sulfidation, original “pristine” ligands (molecular groups or ions used to functionalize particles) were removed or modified so that silver sulphide emerged as the new ligand shell. By studying silver nanoparticles functionalized with two common polymeric ligands – PVP and thiolated PEG – Lau and his students found that silver sulphide formation is ligand-dependent and essential for the persistence of silver as nanoparticles (2018 Env. Sci.: Nano 5 1090). They found that sulphidation helped to immobilize PVP-functionalized silver nanoparticles so that they stick to sand or sediment surfaces more readily, decreasing the chances of exposure. On the other hand, sulphidation made PEG-functionalized silver nanoparticles more mobile, although the silver sulphide formed was less toxic. “In other words, two types of silver nanoparticles (same size), could have more than an order of magnitude difference in their persistence in the environment, simply due to a different choice of ligand,” says Lau.
The work of Lau, Mitrano and others is part of a growing body of evidence that investigators need to consider a nanomaterial’s whole life cycle – from synthesis to disposal – before determining its safety. “Relatively simple nanoscale materials can have complex behaviours even in carefully controlled environments,” says Lau, ticking off a long list of factors – including pH, ionic strength and redox conditions – that may affect how nanoparticles evolve. When it comes to fully de-convoluting the complex phenomena that occur whenever nanomaterials interact with their environment, he says, “we still have quite a long way to go”.
- Enjoy the rest of the 2019 Physics World Focus on Nanotechnology & Nanomaterials in our digital magazine or via the Physics World app for any iOS or Android smartphone or tablet.



