Alexander Fleming’s 1928 isolation of penicillin provided a gear change in combatting infectious disease, progressing from folklore preparations of medicinal moulds to the barrage of purified drugs in use today. Yet as bacterial strains evolve a resistance to antibiotics, fatal infectious diseases could once again be on the rise, with microbial infection set to become a bigger killer than cancer by 2050. By clamping down on drug efflux processes in cells and providing alternative bactericidal agents, nanotechnology is showing great promise for fending off this microbial armageddon

Today’s widespread use of antibiotics began with 19th century studies of fungal and bacterial colonies, which led to the idea of harnessing the antagonistic substances released by competing microorganisms to defend humans against them. However, silver also has antimicrobial effects that have long been exploited, from its use in cutlery and tableware to more medicinal purposes in wound dressings. Yet an understanding of the mechanism behind silver’s antimicrobial activity is only recently coming to light. “The bactericidal effects of the nanoparticles are size-dependent,” concluded a 2004 report in Nanotechnology by Miguel Jose Yacaman and colleagues at the University of Texas at Austin in the US and Centro de Investigaciones y de Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV-IPN) in Mexico. They had investigated the antimicrobial activity of silver nanoparticles sized 1–100 nm and found that only nanoparticles sized 1–10nm presented a direct preferential interaction with the bacteria.
The result was seminal at the time. However, more recent studies, including a report by Pedro J J Alvarez and colleagues at Rice University in 2012, have suggested that the bactericidal activity stems from the presence of silver ions as opposed to the nanoparticles themselves. “We rule out direct particle-specific biological effects by showing the lack of toxicity of AgNPs [silver nanoparticles] when synthesized and tested under strictly anaerobic conditions that preclude Ag(0) oxidation and Ag+ release,” Alvarez and colleagues report. Their work suggests that apparent size and morphological dependences are due to the higher surface areas giving rise to higher ion concentrations. Not only nanoparticles of silver itself, but even bacteria that have died from silver exposure can relay silver’s cytotoxicity. This kind of “zombie effect”, as the ingested silver in dead bacteria proves fatal to other living bacteria that come into contact with it, was reported in Scientific Reports in 2015.
Enhancing the alternatives
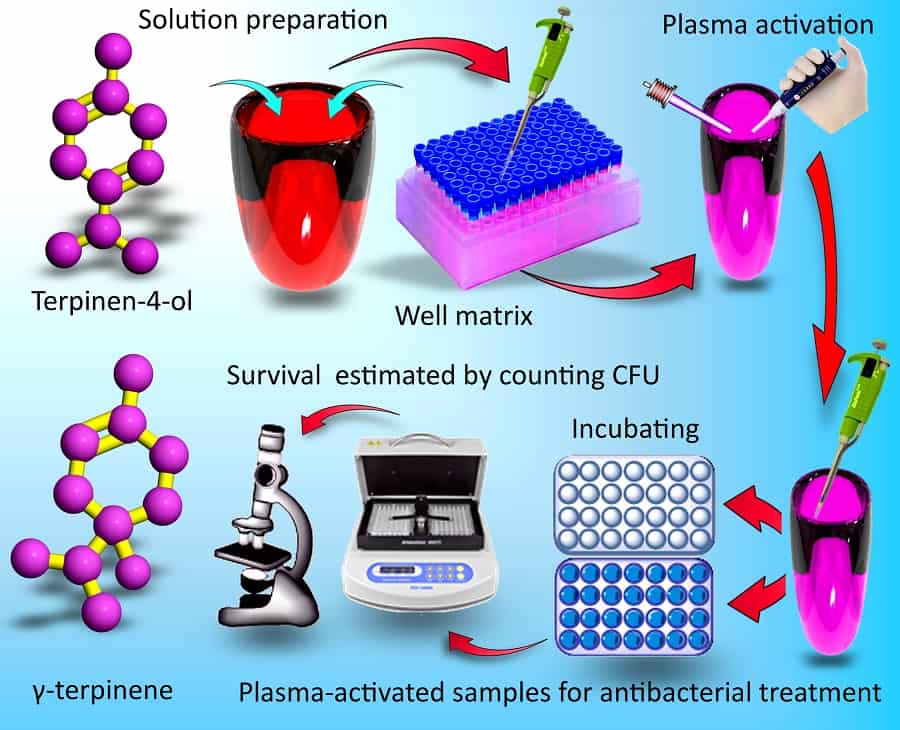
Plants have their own defence mechanisms to combat microbial infections. The primary metabolism of plants caters for all the basic needs for growth and seed production. But alongside this there are secondary metabolic processes that interact with other organisms and the outside world, for example encouraging pollinators or deterring pathogens. The small molecules plants produce in secondary metabolic processes to deter pathogens may seem the perfect alternative to the antibiotic substances produced by competing microorganisms. Discouragingly, at dosage levels that safely avoid adverse side effects, the antibiotic activity of these plant secondary metabolites (PSMs) as they naturally occur is insufficient to combat disease. However, reporting in Nano Futures earlier in 2017, Kateryna Bazaka and colleagues in Australia, Singapore and the US have now shown that plasma treatments can effectively enhance PSM antibiotic activity to useful levels. While further work is required, the researchers hypothesize that the enhanced biological activity stems from chemically active oxygenated derivatives.
There have been reports of other approaches to enhance the microbicidal activity of PSMs, but they tend to be complex and specific to a particular PSM. In contrast, the generic plasma treatment could potentially be applied to various other substances to provide a greatly extended range of alternatives to traditional antibiotics. Bazaka and colleagues have not only demonstrated enhanced activity in PSMs with existing microbicidal activity, but also PSMs with negligible activity. In fact the plasma-enhanced effects are so generic that a level of antimicrobial activity can even be achieved with water. “When this plasma-activated water was used in conjunction with other drugs, the treatment outcome was enhanced, even though in those experiments the drugs were not treated by plasma directly,” Bazaka told nanotechweb.org. The plasma device used in the study is already certified in Europe to be used by medical and veterinary practitioners for wound treatment, bringing the approach still closer to implementation for fighting disease.

Working in synergy
Besides offering alternatives to antibiotic drugs, nanostructures have also demonstrated an ability to clamp down the main microbial cell defence mechanism against antibiotics – the efflux pump. Vincent Rotello, professor at the University of Massachusetts at Amherst, has led research into the symbiotic effect of delivering gold nanoparticles with antibiotics, reported in Nano Futures earlier in 2017. “We realized this was a way to resurrect antibiotics that bacteria have become resistant to,” he told nanotechweb.org. While nanomaterials tend to lyse cell membranes indiscriminately, combining with antibiotics helps the more targeted drug action to get past the efflux pump defence that bacteria use to flush drugs out.
The team functionalized the gold nanoparticles with hydrophobic ligands that lock in with the hydrophobic cell membrane, thereby maximizing the nanoparticles’ effect. The nanoparticles also repress other transporter proteins crucial for the survival of the bacteria, further aiding the role of the antibiotics. The researchers demonstrated that combined with the functionalized gold nanoparticles, the minimum concentration of the drugs levofloxacin and ciprofloxacin needed to inhibit E. coli was decreased by a factor of 16. As Rotello points out, this equates to the difference between “eating it like cereal and eating a pill”.
While there are significant differences in strategy needed in treating infectious disease and fighting cancer, both strands of medicine currently suffer from evolved drug resistance that stems from the same efflux pump action in a cell’s defence. While Rotello’s group was able to shut this down with functionalized nanoparticles, groups working in physical oncology have adopted a genetic approach. Leu-wei Lo and colleagues in Taiwan used microporous silicon nanoparticles to co-deliver the anticancer drug doxorubicin with DNAzyme, which targets the transcription factor for the multidrug resistant proteins that cells produce to flush drugs out. They report the work in Nano Futures. Crucially, the microporous silicon nanoparticle allows sequential release of the genetic and chemotherapeutic drugs, so that the DNAzyme has time to cleave the transcription factor and inhibit expression of the efflux proteins before the doxorubicin is released. Since the transcription factors targeted are key to cell growth, metastasis of the cancer was also inhibited.
Antibiotics have been so effective against microorganisms that some infectious diseases have been effectively eradicated, and invasive surgery can be performed relatively safely. It is possible that either through the alternatives or the synergies they offer, nanomaterials may contribute to our continued protection from microorganisms in the years to come. Tragically the rise of antibiotics came too late for Ernest Duchesne, who submitted one of the first scholarly works on the anti-microbial activities of moulds in 1897 before succumbing to tuberculosis. It would be more tragic still if the rise of drug-resistant strains of bacteria left us as vulnerable to bacterial infections as we were 130 years ago.



