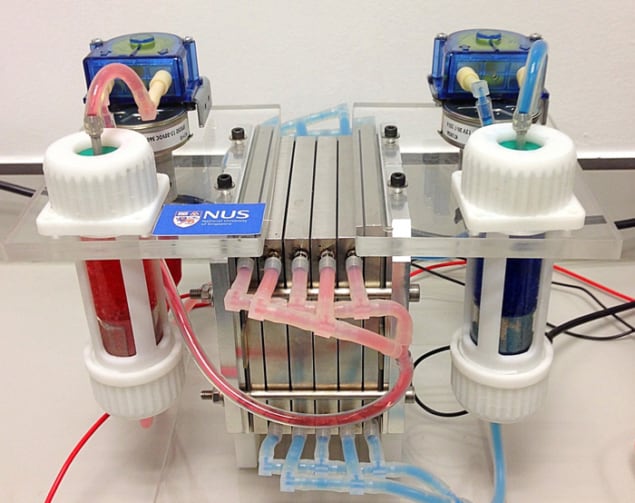
Redox-flow batteries could be very useful for the safe storage of excess energy in electricity grids, but their deployment has been held back because they have far lower energy capacities than conventional lithium-ion batteries. Now, researchers in Singapore have built a new type of redox-flow battery that offers a higher energy capacity without losing the safety advantages that such batteries bring.
As more electricity is generated from renewable sources, electricity suppliers will have to find efficient ways of storing energy produced when the Sun is shining (or the wind is blowing) for use at times of peak demand. Storing energy in rechargeable batteries is one option, and various technologies that are used today including traditional lead–acid batteries and state-of-the-art lithium-ion batteries. However, these established technologies have their problems. Lead–acid batteries have limited storage capacity and lithium-ion batteries are prone to overheating, which makes the latter unsuitable for use in large-scale facilities.
A redox-flow battery employs liquid electrolytes that are stored in two separate tanks. During charging or discharging, one liquid is circulated around the battery’s anode and the other around its cathode – which are themselves separated by a semipermeable membrane. Such batteries are less prone to overheating and combustion because the energy is stored in the tanks, which can be isolated from the point at which the electrochemical power generation takes place.
Tanks of energy
“It’s a bit like with the internal-combustion engine, where you have a tank for the gasoline and you just pump it into the engine to produce power,” says materials scientist Qing Wang, who led this latest research. The most developed designs use vanadium, which is stored and transported in aqueous solution. Unfortunately, this severely restricts energy capacity, because the vanadium salts are not very soluble in water.
Wang and colleagues have developed a new type of redox-flow battery in which the cathodic tank contains lithium–iron-phosphate granules and the anodic tank contains granules of titanium dioxide. When the battery is charged, “redox mediators” dissolved in the electrolyte are pumped through both tanks. Under the influence of an applied voltage, one of the redox mediators oxidizes the lithium in the tank, transporting the lithium ions into the reaction vessel. The reaction vessel is divided by a partially permeable membrane that allows lithium ions to pass but not the redox mediators. In the anodic half of the reaction vessel, other redox mediators combine with the lithium ions. These are then pumped through the titanium dioxide, where the lithium ions are reduced back to lithium metal, which intercalates into the titanium dioxide.
When the battery is discharged, the reaction runs in reverse, returning the lithium to the cathode. Because the lithium is stored in solid form in both of the charged and discharged states of the battery, the energy density of the new lithium-flow battery is about 500 Wh/l. This is around 10 times that of a vanadium redox battery.
Better membrane
The researchers are now optimizing their new battery with an emphasis on improving the performance of the membrane. Conventional flow batteries use a membrane made from the polymer Nafion, which could not be used in the new battery because it lets through too many redox-mediator molecules. Wang and colleagues solved this problem by combining Nafion with the polymer PVDF, which stopped the redox mediators from passing through. However, this new membrane also restricts the lithium-ion flow, which reduced the charge/discharge rate of the battery. For grid storage, Wang says the permeability “works fine, but it’s not perfect”. If it can be improved further, Wang says, the battery could also be useful for electric vehicles – although he concludes that “there’s quite a long distance to go”.
Michael Aziz of Harvard University describes the work as “very innovative”, but he is sceptical of Wang’s claim that the charge/discharge rate is adequate for grid storage, saying that it is 10,000 times lower than that of an aqueous vanadium redox-flow battery. He points out that Nafion is very expensive and a practical battery would need 10,000 times the amount of membrane to achieve the same performance as aqueous vanadium. “For grid storage, it’s more important that a battery be cheap than that it take up a very small area,” he explains.
John Goodenough of the University of Texas at Austin – the inventor of the lithium-ion battery – agrees. “I don’t think they have the answer to the least-expensive battery for large-capacity electrical-energy storage,” he says, “but the approach may prove feasible one day.”
The new battery is described in Science Advances.