Researchers from China and the USA have developed a novel bioink that can efficiently bioprint, culture and expand human induced pluripotent stem cells (hiPSCs). The discovery offers a novel and powerful tool for the expansion of hiPSCs for applications such as biofabrication and tissue engineering (Biofabrication 10 044101).
HiPSCs can be generated from adult cells of any individual and can be subsequently turned into any cell type in the body. This is reminiscent of the ability of embryonic stem cells, but hiPSCs avoid rejection by the patient’s immune system (as they are derived from the patient’s own cells) and are not subject to the same ethical concerns. As a result, hiPSCs have generated considerable hype as a means to create bespoke stem cell or tissue-engineered therapies for patients.
For this to be realised, however, hiPSCs have to be scaled up substantially. The cell numbers required to generate micro-tissues in the laboratory range from 100 million to one billion cells. To repair solid tissue or organs, this figure would be closer to 10-100 billion cells, which is no small task economically or practically.
Current culture methods for hiPSCs are mainly conducted in two dimensions, where expansion efficiency is poor, and cells may begin spontaneously maturing into adult cells. Therefore, generating many millions or billions of hiPSCs is a slow process, which is unacceptable when a patient may require them in a timely manner.
To overcome this, researchers led by Wei Sun at Tsinghau University and Drexel University have devised a novel, three-dimensional scaffold to support the culture and proliferation of hiPSCs in a more biomimetic environment. The scaffold is composed of hydroxypropyl chitin (HPCH) and Matrigel. HPCH can be gelled and liquefied by changing its temperature and operates as the scaffold “backbone”, while Matrigel is a known bio-supportive material that promotes cell adhesion inside the scaffold.
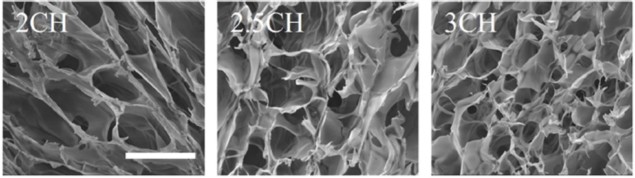
While encapsulation and 3D culture of hiPSCs has been performed previously, the HPCH scaffold has unique properties. As it only requires temperature change and not additional components to cross-link the gel, constructs are more homogenous and cell viability is not affected. The concentration of HPCH required to gel is also low, allowing a highly porous material that is ideal for cell proliferation and nutrient exchange.
Bioprinting hiPSCs has been notoriously difficult as they are fragile and easily damaged. Here, by tightly controlling printing parameters such as print speed and nozzle diameter, the researchers were able to define the optimal conditions for maintaining cell viability. They also assessed a range of HPCH and Matrigel combinations in the bioink to support the cells.
The cells were left for 10 days post-printing and their proliferation was assessed in the various bioink mixtures. Cell growth was higher in the printed, HPCH scaffolds than the scaffold-free or 2D cultures. Interestingly, the researchers also noted that varying the density of HPCH changed the variability and overall size of the cell aggregates that formed: lower concentration gave larger, more variable aggregates; higher concentration gave smaller but more consistently sized aggregates.
Critically, hiPSCs must remain in their pluripotent state while in culture and not mature spontaneously. Pluripotent cells are often cultured on specific substrates to maintain this pluripotency, and the radical shift to a 3D culture can instigate this uncontrolled change. The researchers evaluated the encapsulated cells over 10 days and through comprehensive characterization and showed that the cells retained their pluripotency.
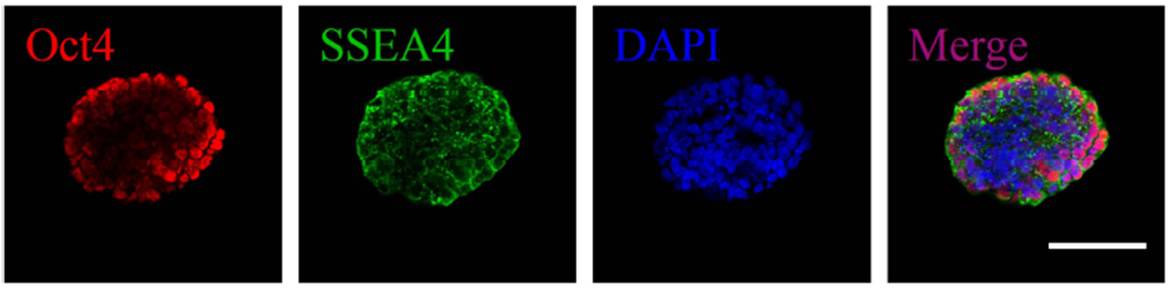
The HPCH/Matrigel bioink could prove to be a boon in the developing field of pluripotent stem cell printing and cell scale-up for tissue engineering. The researchers showed that encapsulated cells maintain viability and pluripotency, and that the gel can be easily dissolved to facilitate culture expansion. Its 3D nature provides a more physiologically relevant environment for cell culture than 2D approaches, while also providing more space for cell growth.
By controlling the bioink composition, aggregates can be formed with high uniformity in size, which is necessary for well controlled maturation. Further investigation into the long-term culture, passage, and upscale of hiPSCs may lead the way to improved micro-tissue fabrication and differentiation.



