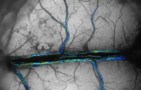
A new model based on the blood-vessel network in a rat brain shows that the vessel position within its circulatory network does not influence the blood flow nor how nutrients are transported. Instead, transport is controlled mostly by the dilation of vessels. As well as providing new insights into the circulatory system, the model could lead to better artificial tissues and brain-scanning techniques – and might even improve the performance of solar panels.
The human circulatory system is a complex network of interconnected blood vessels – arteries, veins and capillaries – that transports oxygen, hormones, nutrients, and waste throughout the body.
In the brain, if a certain neuron needs more nutrients, it will release signalling molecules that will cause the tiny muscles around a nearby blood vessel to relax. This widens (dilates) the vessel, increasing the blood flow and local nutrient availability. Blood flow fluctuations are therefore a sign of an increased brain activity and can be used for scanning brain tissues – using functional magnetic resonance imaging (fMRI).
Important questions
Increasing the blood flow to certain brain regions is complicated from the perspective of flow dynamics and researchers are keen to understand how transport networks adapt to their changing environment. Questions include how exactly does dilation bring more blood to a certain region in the brain? Does the position of the capillary within the entire vessel network influence the blood flow, or is the nutrient transport efficiency only related to the vessel dilation?
To answer these questions, physicists at the Max Planck Institute for Dynamics and Self-Organization in Germany led by Karen Alim created a theoretical model of blood flows in a previously reconstructed brain-vessel network.
Building upon an advanced dataset of the full microvasculature of a rat obtained by two-photon microscopy at David Kleinfeld’s lab at the University of California San Diego, Alim’s team had access to both the dynamic and static properties of the vessel network brain comprising over 20,000 individual vessels. The dataset also included information on the blood flow in these vessels. Using this information, they analysed the nutrient supply dynamics under different scenarios.
The power of dilation
Alim and colleagues found that controlling the nutrient and metabolite supply by vessel dilation is not influenced by the vessel network architecture, nor the position of the respective vessel within the architecture. Since the flow is coupled in these systems, the key role is played by vessel dilation, especially if nutrient and particle transport by advection (fluid motion) is considered.
Alim says that their “findings were quite surprising. The global coupling, so the exact network position of a single vessel that is dilating, does not matter when it comes to nutrient supply.”
“The situation becomes more complicated when it comes to the transport of resources in the flow stream, which is a non-linear problem,” she adds. If a single vessel dilates, it brings more nutrient to its close surrounding, thus depriving the remaining blood of nutrients. This means that downstream of this particular vessel, spatial correlations need to be considered, to accommodate the competition for nutrients between nearby vessels.
Better solar panels
Alim’s results show how all of the events shaping the structure of a network, like in this case a tiny region in a rat brain, can self-organize to produce a high level of control necessary to keep neurons alive and functioning. Her model could provide tools to better understand the design of a functional artificial tissues, or to improve the fMRI process to detect local increase in brain activity.
Insect-inspired microscope takes videos of blood cells in motion
Working with theoretical models has a large advantage of having the possibility to adapt the framework for other applications. The team is also studying an inverse problem of using blood flow to transport heat. Their model organisms are snakes; by looking at the 3D vasculature of a heat-detecting organ, the team aims at understanding the dynamics of these flows and using them in bionic direction to improve the design of solar panels.
Marcus Roper of the University of California Los Angesles UCLA told Physics World that he has seen the “specific data set that Dr. Alim’s group analyzed. Personally, it feels overwhelming, for the number of vessels that it contains. I am amazed that they were able to tease out such a clean and compelling explanation.”
He speculated whether the pathological blood vessel networks created by tumors or the disrupted networks that are re-established in the brain following traumatic brain injury could also possess the self-organization properties reported by Alim’s team. “Obviously, we are a long way from the rubber meeting the road in terms of clinical applications, but I think that understanding physical principles for how the network functions is necessary if we are to use these new data streams to their fullest potential,” he adds.
The research is described in Physical Review Letters.