This year is the centenary of a major event in modern science. So, asks Robert P Crease, where are the celebrations?
“What was the single biggest turning point of modern science?”
My colleague Phil Allen, a condensed-matter theorist, greeted me with that question the other day, suggesting by his tone that obvious answers were incorrect. I tried some anyway.
“Rutherford’s 1911 discovery of the nucleus?” He shook his head. Ernest Rutherford was right in concluding that an atom’s mass resides mostly at its centre, but his model was not taken seriously for two years because orbiting electrons ought to radiate energy and fall into the nucleus, which does not happen.
“The Bohr atom of 1913–1914?” Phil conceded that Niels Bohr’s model was an enormous leap, for it saved Rutherford’s idea by demonstrating that the electrons could not radiate energy and were confined to specific orbits. But it did not work for any atom besides hydrogen because it neglected things such as electron–electron interactions.
“Planck, the quantum, 1900?” But Max Planck initially saw quantization as only a mathematical fix and five years elapsed before someone (Einstein) suggested the quantum governed not oscillators but light itself. It took another decade or so for that idea to spread.
“Compton scattering, 1923?” The finding that light quanta are particle-like in that they carry momentum as well as energy is the subject of a book, The Compton Effect: Turning Point in Physics by Roger Stuewer. But it affected only atomic physics, and Phil was asking about something with an impact on many scientific fields.
I was still drawing a blank when Phil helped me out. “This year’s the centennial,” he said.
It was 100 years ago
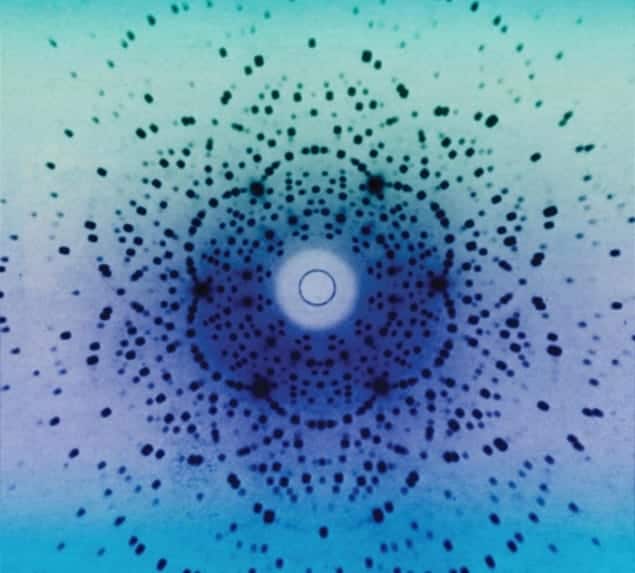
That gave it away: the discovery of X-ray diffraction in crystals, in what Einstein called one of the most beautiful discoveries in physics.
The year 1912 was an ambiguous time in the history of science, when it was not fully certain whether X-rays were electromagnetic waves, or even if crystals consisted of regularly spaced atoms. Early in 1912 the German theoretical physicist Max von Laue – in discussions with a graduate student, Paul Peter Ewald – realized that one way to test some of these ideas was to shoot X-rays through crystals and look for a diffraction pattern.
On 21 April 1912 the experimentalists Walter Friedrich and Paul Knipping irradiated copper-sulphate crystals, large pieces of which were easily available, and soon obtained a pattern of regular points on photographic plates, now called “Laue diagrams”. Laue, Friedrich and Knipping announced the result to the Bavarian Academy of Sciences on 4 May, and two years later Laue won the 1914 Nobel Prize for Physics “for his discovery of the diffraction of X-rays by crystals”.
X-ray diffraction provided two things: a way to find out where atoms in crystals are, and a way to measure X-ray wavelengths, and thus determine frequencies and energies. Suddenly X-rays became a highly productive tool with a huge scope. Spectroscopy, for instance, can only be done by using a crystal as a diffraction grating to provide monochromatic X-rays of known energy. “In the truest sense of the word,” science historian Armin Hermann once wrote, “scientists began to cast light on the structure of matter.”
Events moved swiftly. William Henry Bragg and his son Lawrence masterfully exploited diffraction, discovering “Bragg’s law” and creating the first X-ray spectro-meter, for which they shared the 1915 Nobel prize. Information provided by diffraction enabled Henry Moseley to conduct his famous studies of the X-rays emitted by atoms. The Dutch lawyer and amateur physicist Antonius van den Broek – one of the quirkier figures in nuclear physics who was fascinated by numerical regularities – pointed out that the “atomic number” Z, not the “atomic weight” A, was the key parameter in diffraction, which implied the existence of a positive, charge-carrying particle. In other words, X-ray diffraction proved that Z is the number of electrons in an atom, thereby giving theoretical meaning to Bohr’s picture of the atom.
The critical point
Despite these positives, the discovery of X-ray diffraction is rarely heralded as a turning point in science. Even the venerable Abraham Pais gave it just a one-sentence nod in his authoritative history of 20th-century physics Inward Bound. So why the neglect? One reason is the false impression that X-ray diffraction is a mere technique of crystallography rather than a discovery with a broad and significant impact on physics. Another is our tendency to overvalue theoretical breakthroughs. Finally, condensed-matter physics is not seen as being as “sexy” or fundamental as other areas of physics.
The discovery is, however, familiar to science historians as an example of the fight between “internalists”, who take guidance from memoirs and recollections of participants, and “externalists” who view science history as the province of professional historians. In 1962, on the discovery’s 50th anniversary, Ewald published a book called Fifty Years of X-ray Diffraction that included recollections by participants. Shortly afterwards, the maverick historian Paul Forman attacked science history based purely on recollections, which he said tended to involve “gross misrepresentations” that neaten conceptualizations and purify motives (1969 Arch. Hist. Exact Sci. 6 38). Ewald’s reply blasted Forman’s article as “worthless except as an example to which conclusions the study of literature under a biased point of view can lead” – the bias being a desire to prove the thesis that the scientists were self-aggrandizing. The exchange in turn inspired the journal’s editor, a historian, to remark that “physicists are as pig-headed and superstitious as any monk of the dark ages”.
These disputes apart, it is clear to me that the discovery of X-ray diffraction was a central event in modern science. “That discovery brought it all together,” said Phil. “It suddenly made the atom real and accessible to accurate measurement, showed us what it ‘looked like’ – and how it was arranged in molecules and solids. For me, the year 1912 in science was the amazing turning point.”



