
Dutch researchers have engineered 3D cellular constructs called organoids for kidney research using human induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs). The organoids were successfully vascularized once implanted in vivo (Stem Cell Reports 10 751).
The kidney has multiple functions: homeostasis (balance of many body functions), control of blood pressure, production of red blood cells, and elimination of waste (from food, medication or toxic substances), while keeping a balance of essential substances, fluids and minerals in the body.
One kidney is composed of more than 1 million nephrons, the functional unit of the kidney. The nephron filters the blood, processes nutrients and passes out waste from the blood. The blood is first filtered by the capillary network (glomerulus). The filtrate is collected in the Bowman’s capsule and passes through a series of renal tubules (proximal tube, loop of Henle and distal tube). These absorb water, minerals and glucose. Filtered fluids leave the nephron by the collecting duct and enter the renal pelvis.
The cells composing the kidney can be developed in the lab. However, the classic way to cultivate cells in vitro is using a 2D structure, which does not represent the complexity of the organ. Instead, researchers are investigating ways to cultivate cells in a 3D system, as organoids. Organoids are cellular 3D constructs that replicate fully or partially the structure, cellular organization and composition of the in vivo organ. One limitation of organoids is their incapacity to undergo morphogenesis, meaning that they can’t be physically modified to achieve a complete maturation. For example, functional vascularization is not achievable yet.
Now, Ton Rabelink and his team at the Leiden University Medical Center in the Netherlands have developed a kidney organoid which, once implanted in vivo, becomes vascularized and starts to become more mature.
The researchers were able to generate kidney organoids from ESCs and iPSCs obtained by reprogramming of somatic cells to the pluripotent state. Both cell types were able to develop into kidney organoids.

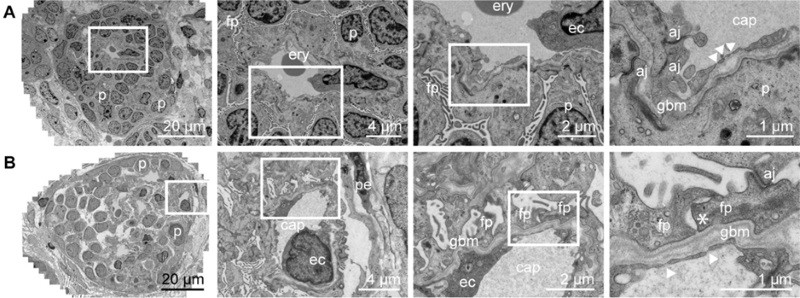
They identified many structures in these kidney organoids, including the glomerulus surrounded by the Bowman’s capsule, the renal tubules and the collecting duct. They also observed more specific features, including the presence of podocytes in the glomerulus (cells involved in the retention of plasma proteins from going into the urine), interstitial cells, which act as the kidney scaffold, endothelial cells (that form blood vessels) and pericytes, which are cells localized around the vessels.
Upon implantation of the organoid under the renal capsule of mice, the researchers observed glomerular vascularization by the mouse endothelial cells, without needing further stimulation. Also, organoid maturation and organization was more advanced upon transplantation in vivo, compared with prolonged culture in vitro.
This study presents the development of a more mature kidney organoid that is more comparable to adult kidneys. This research will be useful in many applications, such as drug screening, disease modelling and studying kidney regeneration.



