For patients with early-stage breast cancer, breast-conservation surgery is a preferred option to mastectomy. However, if histopathological analysis reveals that the malignant tissue was not totally removed, the patient will need to undergo re-excision days later. A tool that could accurately assess tumour margins during breast-conserving surgery would help surgeons to totally remove the tumour in the initial procedure and reduce re-excision rates, which are currently up to 30%.
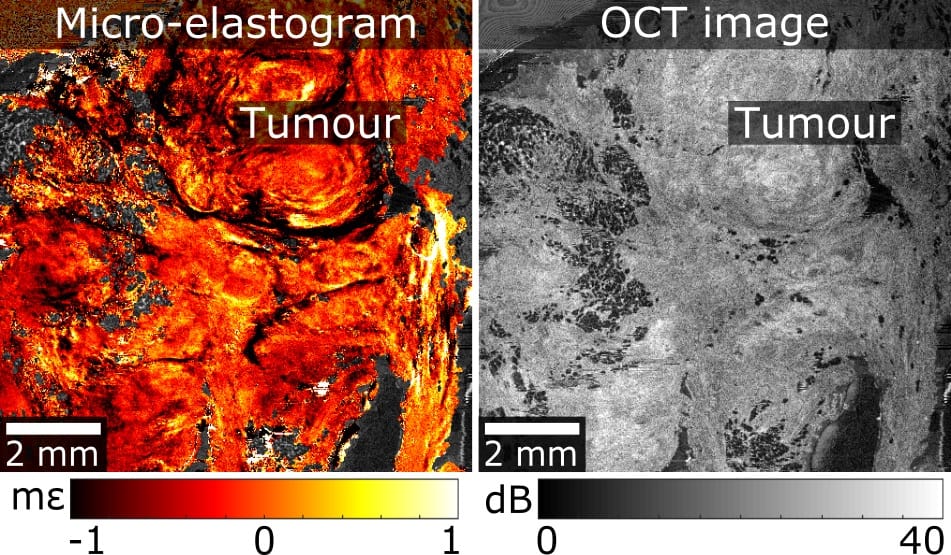
A technique that shows promise to visualize tumours in human breast tissue is optical coherence micro-elastography (OCME). Researchers at the University of Western Australia have used OCME to visualize a range of tumour morphologies in wide-local excision (WLE) specimens removed during breast-conservation surgery, and determined its potential for imaging tumour margins (Biomed. Opt. Express 10.1364/BOE.9.006331).
OCME, a variant of optical coherence elastography, is a label-free imaging technique that generates mechanical contrast by mapping the deformation (local axial strain) that results from compressing the excised tissue. As OCME utilizes phase-sensitive optical coherence tomography (OCT) to measure local strain, it also generates optical contrast and can produce dual-contrast images, called micro-elastograms, by fusing together mechanical and optical contrast. Micro-elastograms improve the ability to detect a broader range of tumour morphologies compared with OCT images alone.
The OCME system used for this research comprises an OCT system, a specimen mounting mechanism, and a wide-field translation system that generates images of approximately 46 x 46 mm. An automated algorithm segments the wide-field OCT scans into dense and non-dense regions, and generates individual wide-field en face micro-elastograms in 20–30 s, a critical ability for rapid interpretation in eventual clinical use.
Led by principal investigator Brendan Kennedy, researchers at the BRITElab of the Harry Perkins Institute of Medical Research acquired OCME data sets of 28 margins from 17 WLE specimens. Of the 28 margins scanned, 23 were clear and consisted of adipose tissue and stroma, while five contained tumour within 1 mm of the edges. As OCME provides optical and mechanical contrast from a single dataset, it can generate OCT images alongside micro-elastograms.
Clinical transfer
The researchers identified several challenges to be resolved before OCME can be used clinically. These include incorporating methods to further increase contrast across a range of tumour types, including highly cellular tumour, for example. The authors also need to gain better understanding of the effects on mechanical properties caused by thermal damage to the tissue, which can occur during excision.
In addition, a much faster scanning protocol needs to be developed to reduce imaging time. The current automated protocol scans a maximum of two margins in approximately 30 minutes. The post-processing time of data sets, which takes approximately three hours per margin, also needs to be significantly reduced.
In ongoing work, the team has incorporated quantitative micro-elastography, another variant of optical coherence elastography, into the system to enable a broader range of tumours to be visualized by quantifying tissue stiffness. “Using this new system, our research team is in the final stages of a larger study in which we have scanned over 140 margins from 71 patients at Fiona Stanley Hospital in Murdoch, Western Australia,” lead author Wes Allen tells Physics World. “The micro-elastograms generated will allow a blinded reader study to be performed, determining the diagnostic accuracy.”
The team is also working closely with Christobel Saunders, a consultant breast cancer surgeon, senior pathologist Bruce Latham and a start-up company from the University, OncoRes Medical, of which Kennedy is the chief scientific officer. Allen says that they are working to miniaturize the technology into a hand-held probe that will allow a surgeon to scan the margin of the excised tissue for tumour, as well as directly detecting tumour that has been left in the patient by scanning the breast cavity.



