Transcranial focused ultrasound is being developed as a potential treatment for various brain diseases and disorders. One big challenge, however, is focusing the ultrasound through the skull, which can blur, attenuate and shift the beam. To minimize these effects, researchers at Zeta Surgical have developed an algorithm that automatically determines the optimal location to place a single-element focused transducer.
For therapeutic applications – including, for example, thermal ablation, drug delivery, disruption of the blood–brain barrier and neuromodulation – the ultrasound beam must be focused onto a small spot in the brain. The resulting high acoustic pressure at this spot generates a high temperature or mechanical force to treat the targeted tissues, ideally while avoiding overheating of nearby healthy tissues.
Unfortunately, when the ultrasound beam passes through the skull, which is a complex layered structure, it is both attenuated and distorted. This decreases the acoustic pressure at the focus, defocusing the beam and shifting the focus position.
Ultrasound arrays with multiple elements can compensate for such aberrations by controlling the individual array elements. But cost constraints mean that most applications still use single-element focused transducers, for which such compensation is difficult. This can result in ineffective or even unsafe treatments. What’s needed is a method that finds the optimal position to place a single-element focused ultrasound transducer such that defocusing and focus shift are minimized.
Raahil Sha and colleagues have come up with a way to do just this, using an optimization algorithm that simulates the ultrasound field through the skull. Using the k-Wave MATLAB toolbox, the algorithm simulates ultrasound fields generated within the skull cavity with the transducer placed at different locations. It then analyses the calculated fields to quantify the defocusing and focus shift.
The algorithm starts by loading a patient CT scan, which provides information on the density, speed of sound, absorption, geometry and porosity of the skull. It then defines the centre point of the target as the origin and the centre of a single-element 0.5 MHz transducer as the initial transducer location, and determines the initial values of the normalized peak-negative pressure (PNP) and focal volume.
The algorithm then performs a series of rotations of the transducer centre, simulating the PNP and focal volume at each new location. The PNP value is used to quantify the focus shift, with a higher PNP at the focal point representing a smaller shift.
Any change in the focal position is particularly concerning as it can lead to off-target tissue disruption. As such, the algorithm first identifies transducer positions that keep the focus shift below a specified threshold. Within these confines, it then finds the location with the smallest focal volume. This is then output as the optimal location for placing the transducer. In this study, this optimal location had a normalized PNP of 0.966 (higher than the pre-set threshold of 0.95) and a focal volume 6.8% smaller than that without the skull in place.
Focused ultrasound plus plaque-reducing drugs could slow Alzheimer’s progression
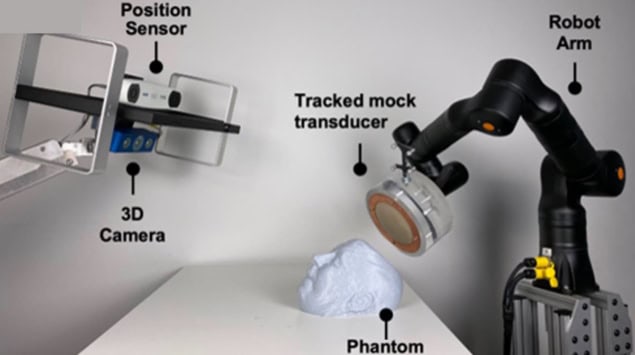
Next, the team used a Zeta neuro-navigation system and a robotic arm to automatically guide a transducer to the optimal location on a head phantom and track the placement accuracy in real time. In 45 independent registration attempts, the surgical robot could position the transducer at the optimal location with a mean position error of 0.0925 mm and a mean trajectory angle error of 0.0650 mm. These low values indicate the potential for accurate transducer placement during treatment.
The researchers conclude that the algorithm can find the optimal transducer location to avoid large focus shift and defocusing. “With the Zeta navigation system, our algorithm can help to make transcranial focused ultrasound treatment safer and more successful,” they write.
The study is reported in Bioengineering.