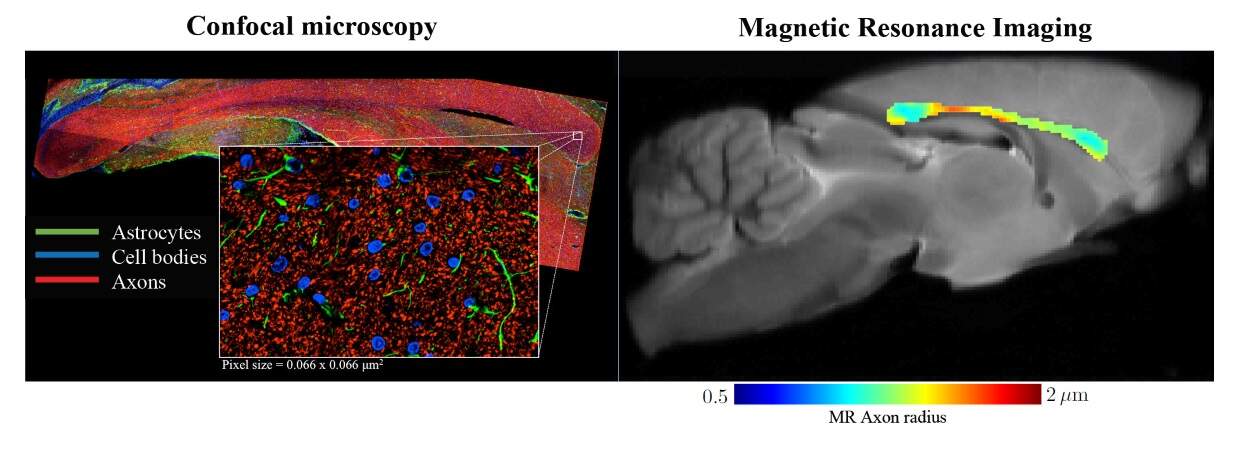
An international team of researchers has established a way to non-invasively measure the radii of axons, fine nerve fibres in the brain, using diffusion MRI (dMRI). Their proposed method showed good agreement with histological studies in both rodents and humans, and outperformed previous techniques in which reported measurements were an order of magnitude larger than histologically-derived axonal sizes (eLife 10.7554/eLife.49855).
Axons are the wire-like protrusions of a neuronal cell, involved in conducting electrical impulses away from the cell body. They are microscopic in diameter and, as bundles, comprise the primary form of communication of the nervous system. Clinical and histological studies have shown that axon radii can range from 0.1 µm to more than 3 µm in the human brain, and that this size, along with myelination, is responsible for the speed of neuronal communication.
In addition, clinical studies of neurodegenerative diseases, such as multiple sclerosis, have revealed preferential damage to smaller axons, while an electron microscopy study involving subjects with autism spectrum disorder showed a significant difference in axon size distribution compared with healthy controls.
Non-invasive axon radii quantification…
Clearly, accurate quantification of axon radius is an important neuroimaging biomarker. This, however, has proven to be a highly challenging task to perform non-invasively. Nevertheless, a team of researchers from the Champalimaud Centre for the Unknown in Portugal, NYU Grossman School of Medicine in the USA and the Cardiff University Brain Research Imaging Centre (CUBRIC) in the UK, set out to achieve just that.
The team used dMRI, a non-invasive and non-ionizing imaging modality that measures the random motion of water molecules in tissues, revealing details of the tissue microarchitecture. The key novelty of the approach is the researchers’ proposed method to separate MRI signals originating from different compartments of the probed tissue. More specifically, they modelled how the water signal behaves in different tissue types, and thereby managed to suppress signal arising from surrounding tissue outside the axons. In doing so, they could more accurately quantify the properties of the axons in the probed brain tissue samples.
…shows unprecedented agreement with histology
To validate their work, the researchers first tested their model on rodents using high-resolution (100 x 100 x 850 µm) dMRI images acquired on a 16.4T MR scanner (Bruker BioSpin). After scanning, they collected 50 µm-thick slices from two rat brains, corresponding to the imaged volume, and stained them to highlight the axons. Finally, they used confocal microscopy to visualize and analyse the tissue slices. Their results showed that the median MR-derived effective axon radius was 3–13% larger than the median radius derived from histology, an error that could be due to shrinkage of the filaments through staining.
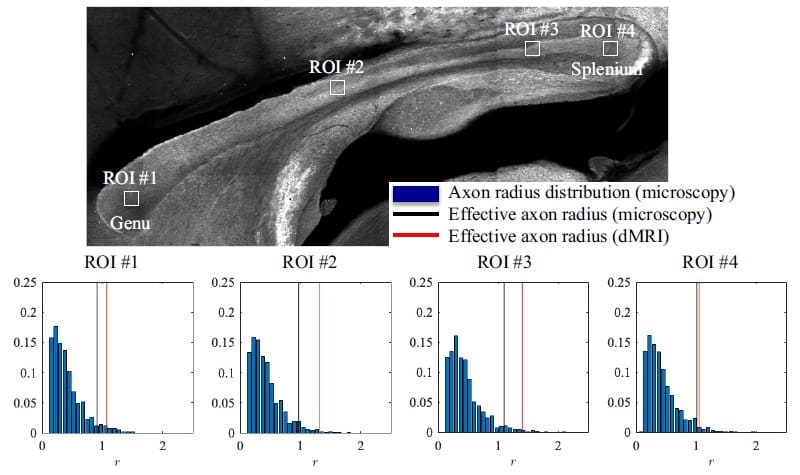
The second part of the study focused on human subjects, who were imaged using a lower resolution (3 x 3 x 3 mm) Siemens Connectom 3T MR scanner. Here, the team used histological values reported in the literature for comparison. Despite this, the researchers showed that their method was able to accurately estimate known axonal sizes with errors an order of magnitude smaller than those in previous MRI studies.
The researchers conclude that their study revealed “a realistic perspective on MR axon radius mapping by showing MR-derived effective radii that have good quantitative agreement with histology”. Thinking about the next steps, first author Jelle Veraart, adds: “The non-invasive quantification of axon diameters using MRI allows clinicians and researchers to identify problems and developmental pathways that arise in the depths of the brain, driving forward treatment and understanding of development and disease progression.”



