Depression is a common disorder, affecting an estimated 5% of adults worldwide, and a leading cause of disability. Although therapy and medications are effective in most patients, there’s a substantial minority who remain resistant to all available treatments.
“It was these patients who really drove us to do this research,” explains Katherine Scangos from the University of California, San Francisco (UCSF). Scangos and colleagues are developing a personalized treatment for severe depression based on deep-brain stimulation (DBS), in which implanted electrodes deliver electrical impulses to targeted structures in the brain.
The researchers have now demonstrated the feasibility of their new approach in a 36-year-old woman with severe, treatment-resistant depression, reporting their findings in Nature Medicine. The patient, Sarah, had previously tried all possible treatment options, including multiple antidepressant combinations and electroconvulsive therapy, with no success at lifting her depression, which restricted her daily life and led to unremitting suicidal impulses.
“When I first received the stimulation, I felt the most intensely joyous sensation and my depression was a distant nightmare for a moment,” said Sarah, speaking at a press briefing. “Months later, when the researchers implanted the chronic device and turned it on for the first time, my life took an immediate upward turn. Now a year into therapy, the device has kept my depression at bay, allowing me to return to my best self and rebuild a life worth living.”
Therapy on demand
The idea of personalized DBS arose some seven years ago in the laboratory of UCSF neurosurgeon Edward Chang, when he observed that stimulating brain areas in epilepsy patients also alleviated emotional symptoms such as anxiety and depression.
While DBS has shown some promise for treating depression, previous clinical trials have revealed highly variable responses between subjects. These inconsistent findings may be due to the use of open-loop DBS, which delivers constant electrical stimulation to a single brain structure. Recent work, however, showed that the effects of DBS are dependent on the emotional state of the patient. In addition, different neural circuits underlie different subsets of depression symptoms and may vary between different people.
“So we set out to see whether we could develop a personalized DBS strategy,” Scangos explains.
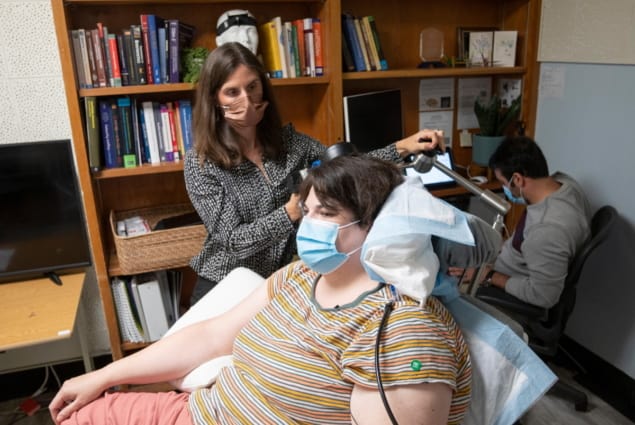
Sarah’s treatment was a two-stage process. To identify her unique depression circuits, the researchers first placed 10 temporary electrodes into various regions of her brain. For 10 days, they continuously recorded neural activity while Sarah rated her symptom severity. This brain mapping approach enabled the team to identify a personalized brain activity biomarker: the finding that high brain activity in the amygdala was correlated with most severe depressive symptoms.
The researchers also used the temporary electrodes to deliver small stimulation pulses to each brain region. They identified an area of the brain, the ventral capsule/ventral striatum (VC/VS), in which electrical stimulation consistently eliminated feelings of depression.
Armed with this information, the researchers then implanted a commercial DBS device (the NeuroPace RNS System) into Sarah’s brain. One of the device’s electrode leads was placed in the amygdala and the other in the VC/VS. They found that a 6 s stimulation at 1 mA was clinically effective and that Sarah could not feel the electrical stimulation at this level.
In addition to identifying a personalized neural biomarker, the other key breakthrough in this work was the use of the biomarker to perform closed-loop therapy, in which stimulation is only delivered when needed. To achieve this, the team programmed the implanted device to continually monitor Sarah’s amygdala for abnormal activity. When this activity was detected (representing a state of severe depression), it automatically triggered a 6 s stimulation pulse to the VC/VS.
The proof-of-concept study proved a notable success. “When we turned this treatment on, our patient’s depression symptoms dissolved and in a remarkably short time, she went into remission,” said Scangos.
Over two months, the device delivered an average of 468 stimulation pulses throughout the day, with few stimulations at night. The team capped the number of stimulations at 300 per day to minimize sleep disturbance from evening therapy.
Ongoing research
Looking at the longer-term implications of this new treatment, Scangos notes that it is too early to tell how long the device will need to remain in a patient, or whether it’s possible that it may somehow help the brain rewire its circuity. However, one advantage of the closed-loop approach is that it does not require continuous stimulation, providing a battery life of over 10 years and enabling the implanted device to provide long-term stimulation if needed.
DBS implant adapts to patient’s neural signals
The researchers also point out that treatments such as cognitive behavioural therapy are almost impossible when a patient is suffering severe depression. If the implanted device can treat the most extreme symptoms, then patients may be more able to employ such therapies. Sarah notes that once the DBS treatment had begun: “I was finally able to use the therapy skills I’d learned and never been able to apply.”
The researchers emphasize that this work is at a very early stage. They cannot determine whether the particular neural biomarker and depression circuit identified in this single-participant study would be present in all individuals. They have now enrolled two other patients in the trial and hope to add nine more.
“These results provide hope that much needed personalized biomarker-based treatment for psychiatric disorders is possible,” says Scangos.




