The recent meeting, Physics-based Contributions to New Medical Techniques, examined how physics technologies are employed to help develop a diverse range of medical applications. One area in particular in which physics has played a vital role is the evolution of particle therapy systems and techniques.
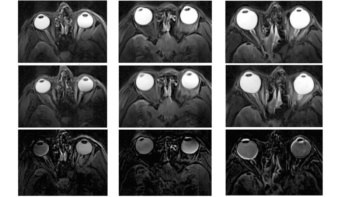
Hywel Owen from the University of Manchester gave meeting attendees an introduction to the UK’s two NHS-funded proton therapy centres, at The Christie in Manchester and UCLH in London. He noted that a number of university academics around the UK are collaborating with these centres to improve the science and technology of radiotherapy. The Christie, which began proton treatments at the end of last year, has also constructed a research beamline at its proton centre, at which researchers from Christie Hospital and the University of Manchester will conduct novel research.
Although the UK centres offer state-of-the-art treatments, Owen explained that there are a number of opportunities to further improve the quality of treatments. Areas in which UK researchers are working include reducing treatment times, improving the imaging and accuracy of treatment, and developing use of alternative particles such as carbon ions and electrons.
Owen described his group’s work to develop the world’s first superconducting cyclotron that operates at 70 MeV. This system aims to provide a route to higher dose rate delivery for shallow treatments such as ocular therapy and will potentially give a better dose distribution than current technologies. The Cockcroft Institute has collaborated with Antaya – one of the world’s leading cyclotron developers – to produce a prototype; the larger magnetic fields obtained with superconducting magnets allow cyclotrons to be made much smaller and cheaper.
Another novel research development is the ProBE (proton boosting extension for imaging and therapy) linac – a joint project between the Cockcroft Institute, The Christie and CERN. ProBE is designed to accelerate protons from a medical cyclotron to the higher energies required for proton imaging. Owen explained that a prototype cavity has been manufactured and is predicted to achieve a gradient of about 54 MV/m. By adding it to a proton therapy centre’s beam transport system, whole-body proton imaging of adults becomes possible.
Rapid QA frees up treatment time
The big advantage of proton therapy arises from the fact that protons deposit most of their energy at a specific depth – the Bragg peak – and then stop, sparing surrounding normal tissue. But as Simon Jolly from University College London (UCL) explained, this highly localized dose deposition is also a disadvantage, as any range uncertainties necessitate the use of margins around the target volume. Effective quality assurance (QA) of proton therapy set-ups is thus essential to exploit the full dosimetric benefit of proton therapy. Unfortunately, such procedures can be time consuming.
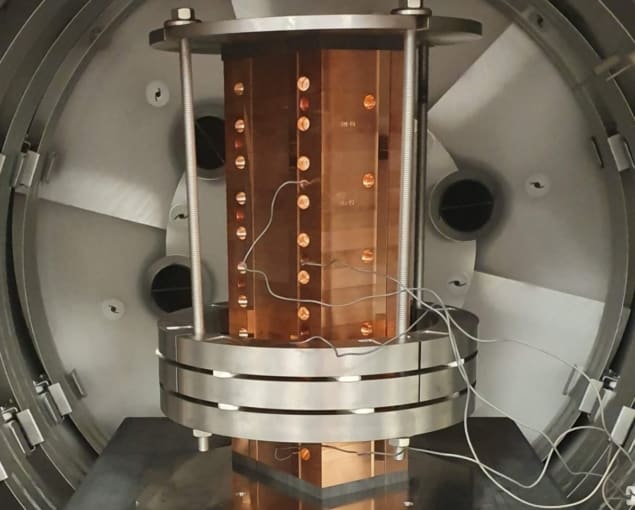
Jolly described a prototype range measurement device under development at UCL that should enable faster and more accurate proton range measurements, thereby speeding up the daily QA process. “We are transferring technology from pure high-energy physics research to proton therapy,” he told the audience.

Multilayer ionization chambers (MLICs) can perform beam range measurements in just a few minutes, but can be bulky and expensive. The UCL device is similar to an MLIC design, but replaces the stack of ionization chambers with individual sheets of plastic scintillator, of the type used in the SuperNEMO double-β decay experiment. Jolly notes that this lightweight plastic is near-water-equivalent, provides high light output and has excellent energy resolution. Unlike MLICs, it is also capable of making measurements at FLASH dose rates.
To measure range, the proton beam is fired horizontally into the end of the stack of scintillator sheets. The device reads out the light signal from each individual sheet using a pixelated sensor placed on top of the stack (over the sheet edges). Beam range can then be estimated from the measured light dose distribution. The system is calibrated by shooting a high-energy proton beam through the entire stack in both directions.
Jolly and colleagues tested the prototype device at several sites, including MedAustron, the Heidelberg Ion Beam Therapy Center and the Birmingham Cyclotron, where it performed its first Bragg peak measurement last March. The system demonstrated a proton range reconstruction accuracy of about 100 µm, well below the clinical requirement of 1 mm.
The team also verified the radiation hardness of the prototype system by performing a “fry up”, at the Clatterbridge Cancer Centre. After continuous irradiation for an entire day, the device showed less than 5% reduction in peak light output and no change in range accuracy. “The detector survived almost 6500 Gy, about a year’s worth of dose,” Jolly noted. “The next step is to build a system for the clinic that is self-contained, easy to use and robust.”