One major challenge when delivering proton therapy is uncertainty in the range of a clinical proton beam travelling through the various tissues and organs in the body. To address this problem, US researchers are developing a system for proton CT and radiography, which could be used both for image guidance and estimation of proton beam range in the clinic. They have now presented the first proton radiographs using their system, including the first real images of biological materials (J. Radiat. Oncol. 10.1007/s13566-019-00376-0).
Currently, proton therapy is planned using X-ray-based CT. The CT data are also used to create digitally reconstructed radiographs (DRRs) for use in daily patient set-up, by comparing the DRRs to X-ray images recorded immediately prior to treatment. This approach, however, introduces inherent inaccuracies due to the need to convert CT Hounsfield units into proton relative stopping power.
The research team – from Loyola University, Proton VDA, the Chicago Proton Center and Northern Illinois University – instead propose the use of proton CT for treatment planning. Proton CT measures proton stopping power directly, significantly reducing beam range uncertainties during planning.
In analogy to the creation of DRRs, the proton CT data can also be used to generate proton DRRs. These pDRRs could someday replace X-rays for high-precision patient alignment in the daily image-guidance process, as well as providing proton range information. Notably, the estimated radiation dose to the patient is only around 1% of the absorbed dose delivered by the X-ray-based approach.
The team has developed a compact proton imaging set-up based on plastic fast scintillation 2D tracking detectors, which detect individual protons in two dimensions, and a photomultiplier tube-based residual range detector. The 2D proton detectors are placed proximal and distal to the patient, while the range detector is located beyond the distal tracking detector.
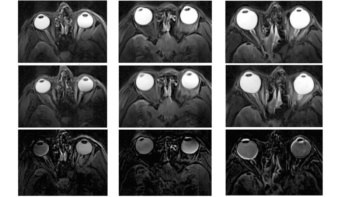
To assess their system, the researchers used the Geant4 Monte Carlo algorithm to create a series of pDRRs from patient X-ray CT data. Three physicians then evaluated the image quality of these idealized pDRRs. All three felt that the pDRRs showed sufficient anatomical detail for patient alignment.
“If the pDRRs were derived from proton CT data, the results would be very similar,” explains senior author James Welsh. “But the pDRRs from proton CT data would be more accurate, since the proton stopping power is measured directly.”

The researchers also obtained the first actual proton radiographs of a biological specimen using their system. Images of a frozen tilapia cichlid fish revealed the fish’s internal bony anatomy. The high level of detail suggests that human bony anatomy will be similarly evident using the system, with images useable for daily image-guidance. Welsh notes that the team has also recently obtained its first proton CT images.
Image reconstruction
In a separate publication, Welsh and colleagues present an image reconstruction system for the prototype proton radiography system. They used Geant4 to simulate raw data detected by the device and wrote dedicated software – pRad – to process these data and reconstruct radiographs (J. Radiat. Oncol. 10.1007/s13566-019-00387-x).

The researchers simulated proton pencil-beam irradiation of a paediatric head phantom. They used the pRad software to reconstruct proton radiographs showing the 2D distribution of water-equivalent path length (WEPL) values. Using a desktop computer with a single CPU and a single graphics processing unit, the software took about 11 s to reconstruct a radiograph from 7.6 million protons.
The team generated radiographs using an iterative reconstruction algorithm, plus two fast non-iterative methods – straight-line projection (SLP) binning and most-likely paths (MLP) binning – and compared the results with a “ground truth” image. They also compared three methods for defining the hull (the surface of the imaged object): one using the known geometry of the object, and two using WEPL to estimate the hull.
Most features in the ground truth image were visible in the reconstructed radiographs, with departures from truth mainly occurring near the outer edges of the object. Radiographs reconstructed with a known hull showed sharper outlines, while simple SLP binning gave the worst results, regardless of hull method used. In all iterative reconstruction and MLP binning cases, the mean WEPL error in the head phantom radiograph was less than 1 mm.
The researchers also simulated proton imaging with intentional misalignments (lateral shifts or rotations) and reconstructed the simulated data with and without alignment correction. The latter illustrated how bad reconstructions arise from misaligned data, while the former demonstrated the effectiveness of the alignment-correction algorithm.
“While there are many challenges with respect to both hardware and software, our prototype is clinically practical enough that we have been able to form a partnership with an interested industrial partner (Cosylab), with the goal of achieving integration into proton therapy treatment rooms,” Welsh tells Physics World. “We are now designing the clinical version and extending automatic image reconstruction to proton CT as well as proton radiography.”