
A survey of eight large European imaging facilities with extensive clinical experience of PET/MRI has found wide variations in daily quality control and assurance procedures that ensure their proper function and image quality. The international study was published on 24 September in Frontiers in Physics.
One reason for the differences in performance testing protocols, the researchers suggested, is the lack of dedicated quality control recommendations for the hybrid imaging modality beyond standard vendor guidelines.
“The reported variations of local PET and MRI quality control procedures and testing frequencies are assumed to partly reflect the variations seen in the existing guidelines for the single modalities and the nonexistence of specific recommendations for PET/MRI,” wrote the authors, led by Alejandra Valladares, a doctoral candidate from the Medical University of Vienna.
“However, in part, the variability in the reporting seems to be caused by differences in the definition of the specific tests between the centres and a lack of in-depth knowledge about the implemented quality control measures included in the daily quality assurance and the tests performed by the vendor during the preventive maintenance,” they explained.
Hybrid hype
The term “hybrid” is more than simply integrating two imaging modalities. It also is the acronym for Healthcare Yearns for Bright Researchers for Imaging Data (HYBRID), a training network project funded by the European Commission (MSCA ITN 764458) with goals that include the promotion of molecular and hybrid imaging modalities to advance personalized medicine and non-invasive disease evaluations. The HYBRID consortium consists of international academic, industrial and non-governmental partners at eight European locations with extensive clinical experience in PET/MRI, along with three PET/MRI vendors (www.hybrid2020.eu).
During the first phase of commercialization almost a decade ago, three PET/MRI systems came to market, all of which had similar technological components and state-of-the-art image reconstruction algorithms, which require stringent quality control procedures and image quality parameters.
“Hybrid imaging systems such as PET/CT or PET/MRI, in particular, present additional challenges caused by differences between the combined modalities,” the authors wrote. “However, despite the increasing use of this hybrid imaging modality in recent years, there are no dedicated quality control recommendations for PET/MRI.”
So, how are institutions handling their duties to keep these hybrid scanners running at peak efficiency? Researchers set out in this study to summarize PET and MRI quality control programs employed by European hybrid imaging sites, as well as guidelines on maintenance and performance measures recommended by the systems’ manufacturers. The end goal was to develop a consensus on minimal quality control standards for PET/MRI for use throughout the HYBRID consortium.
Of the eight facilities with PET/MRI systems, there were five Biograph mMR systems from Siemens Healthineers, two Signa PET/MR systems from GE Healthcare and one Philips Healthcare’s Ingenuity TF PET/MR. The GE and Philips systems both have PET time-of-flight (TOF) capabilities.
Survey results
All PET/MRI centres stated they perform daily quality assurance, which includes assessing detector stability in accordance with the manufacturers’ guidelines. There were, however, cases where facilities handled certain chores less frequently than others (Front. Phys. 10.3389/fphy.2019.00136).
Take sensitivity testing, for example, which was conducted in a “high variability across the centres,” the authors wrote, ranging from “daily, quarterly, annually or at the commissioning of the system (during the acceptance test).” The sensitivity of a PET system reflects “how many events can be detected for a given activity within the field-of-view. A change in sensitivity can possibly be caused by malfunctioning detectors, changes in the energy resolution or the coincidence timing window.”
Regarding spatial resolution, two centres provided no information for how often they tested, while one facility indicated the task was performed daily and the other five participants indicated their evaluations ranged from yearly to once every 10 years.
For image uniformity, three centres indicated they tested daily, compared with three other centres that conducted their assessments every three months, one centre that tested annually, and one location did not perform the test as part of its routine quality control.
As for image quality, attenuation- and scatter-correction accuracy and quantitation, three centres ran those tests annually. One centre reported this assessment every 10 years, two facilities conducted the test as part of its acceptance of the PET/MRI and one centre does not perform it as part of the routine quality control.
Vendor evaluations
The trio of PET/MRI manufacturers also was involved in the survey to describe their standards for quality assurance. For daily quality control of the PET component, all three vendors provide dedicated phantoms containing long-lived positron emitting sources. Using these phantoms, a detector stability test is included within all daily quality assurance procedures, but other system and components testing varied between vendors, the authors noted.
For the MRI component, for instance, Siemens’ quality assurance and preventive maintenance procedures usually includes an image quality test using a spherical head phantom with an outer diameter of 180 mm and a body spherical phantom with an outer diameter of 300 mm, which the company provides. This test is done to assess signal-to-noise ratio (SNR) and artefacts.
GE provides at least one specific phantom in the daily quality assurance package to check the system’s MRI component for its geometric accuracy, ghosting level, and SNR. The imaging centre has the choice of how frequently to perform the test.
While Philips’ daily quality assurance for the Ingenuity PET/MR system does not include any test for the MRI component, the company does provide a 200 mm head phantom to validate the MRI component of its system through an MRI image quality evaluation.
Consensus recommendation
After reviewing the quality control procedures of the imaging centres and the vendors’ guidelines, Valladares and colleagues developed their own set of recommendations.
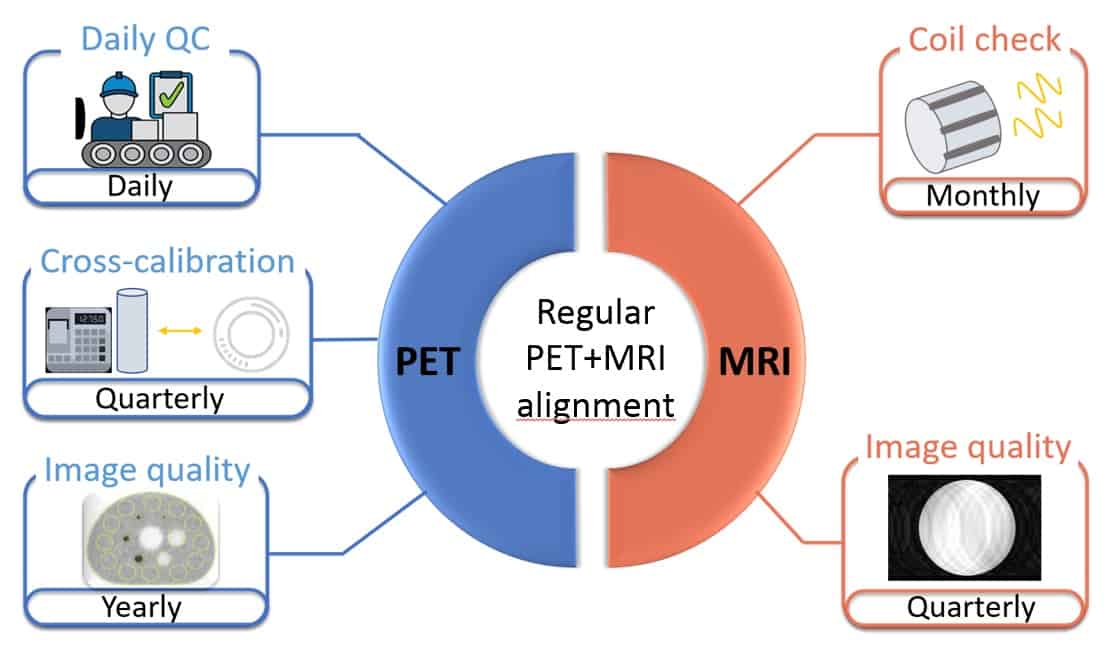
Their minimum consensus for all the three PET/MRI systems includes the following:
- Daily quality assurance implemented by the vendor for the PET component, with quarterly cross-calibration testing and yearly image quality evaluations
- A monthly coil check for the MRI scanner and a quarterly MR image quality test
- An assessment of the PET/MRI system’s alignment after any mechanical adjustments or work on the system’s gantry and after software updates
“It is also recommended to check the function and quality of additional hardware,” the authors added. “Checking the [MRI] coil performance permits the detection of issues with the coils before these affect clinical scans and clinical image quality. This test is particularly important when using flexible coils, which are more susceptible to deterioration.”
Taken together, this consensus recommendation adds positively to overcoming the inertia toward standardized PET/MRI procedures and protocols that will help support high-quality assessments in clinical routine and research using this multiparametric, hybrid imaging modality, they concluded.
- This article was originally published on AuntMinnieEurope.com ©2019 by AuntMinnieEurope.com. Any copying, republication or redistribution of AuntMinnieEurope.com content is expressly prohibited without the prior written consent of AuntMinnieEurope.com.



