With more and more proton therapy centres opening worldwide, proton therapy is now an established cancer treatment option. Clinical treatments are currently planned assuming a relative biological effectiveness (RBE) of 1.1. However, an intensive debate is ongoing in the proton therapy community as to whether this constant value would be better replaced by a variable RBE.
Numerous in vitro experiments have suggested the existence of a variable RBE along the beam direction. While it’s not clear whether this variability impacts clinical outcome, a recent study of paediatric cancer patients showed that changes in MR images three months after proton therapy correlated with dose and linear energy transfer (LET, one of the main determinants of RBE) – suggesting a variable proton RBE.
To investigate this further, a team headed up at OncoRay has created a radiation response modelling framework to assess RBE variability in clinical proton therapy. The researchers applied their model to four glioma patients treated at University Proton Therapy Dresden (UPTD). Their findings suggest a variable RBE for a clinically relevant endpoint (radiation necrosis in the brain) after proton therapy (Phys. Med. Biol. 10.1088/1361-6560/ab3841).
“Currently, there are innumerable review articles published on this RBE topic. However, we believe that only clear and high-quality clinical patient outcome data can substantially contribute to resolve the clinical RBE question,” says senior author Armin Lühr. “Therefore, a solid analysis and modelling framework was needed.”
Plan simulations
Lühr and colleagues developed a UPTD-specific Monte Carlo model to simulate passive scattering proton treatment plans and predict dose and LET distributions. They used this as the foundation of a radiation response modelling framework for analysing clinical side effects, to investigate RBE in patients receiving proton therapy.
The glioma patients analysed for this study had received adjuvant proton(chemo)therapy, fractionated to a total dose of 60 Gy(RBE), and all subsequently exhibited morphological changes in T1-weighted contrast-enhanced follow-up MR images. Such changes can indicate normal tissue complications and are thought to be correlated with radiation necrosis.
The researchers created a dose verification method in which the dose from Monte Carlo patient simulations was normalized to water phantom dose calculations by the clinical treatment planning system (TPS). They simulated the four patients’ treatment plans and compared the simulated dose distributions with TPS calculations.
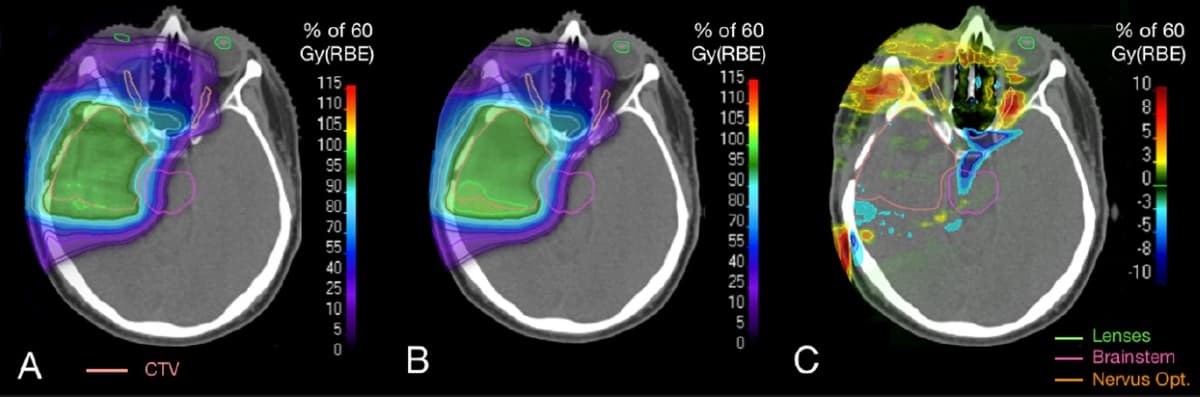
The simulated clinical target volume (CTV) mean dose was, on average, just 0.58% less than the planned CTV mean dose, with a maximum difference of 1.98% — demonstrating that the model reliably characterizes the treatment fields. Dose differences occurred in particular at distal field edges and regions with high density gradients where the precision of the TPS is limited by its pencil-beam dose algorithm.
Response modelling
Using their Monte Carlo model, the researchers established a radiation response modelling framework. They developed two univariable and two multivariable logistic regression models based on voxel-wise correlation of image changes with dose or track-averaged LET, or combinations of the two.
The researchers used each model to predict image changes for the four glioma patients. The findings agreed well with the observed image changes, which were determined by registering post-treatment MR images to the planning CT scan. The correlation with late brain tissue damage was highest using a combination of dose and LET as predictors, with area-under-the-curve values of up to 0.88 in leave-one-out cross validation.
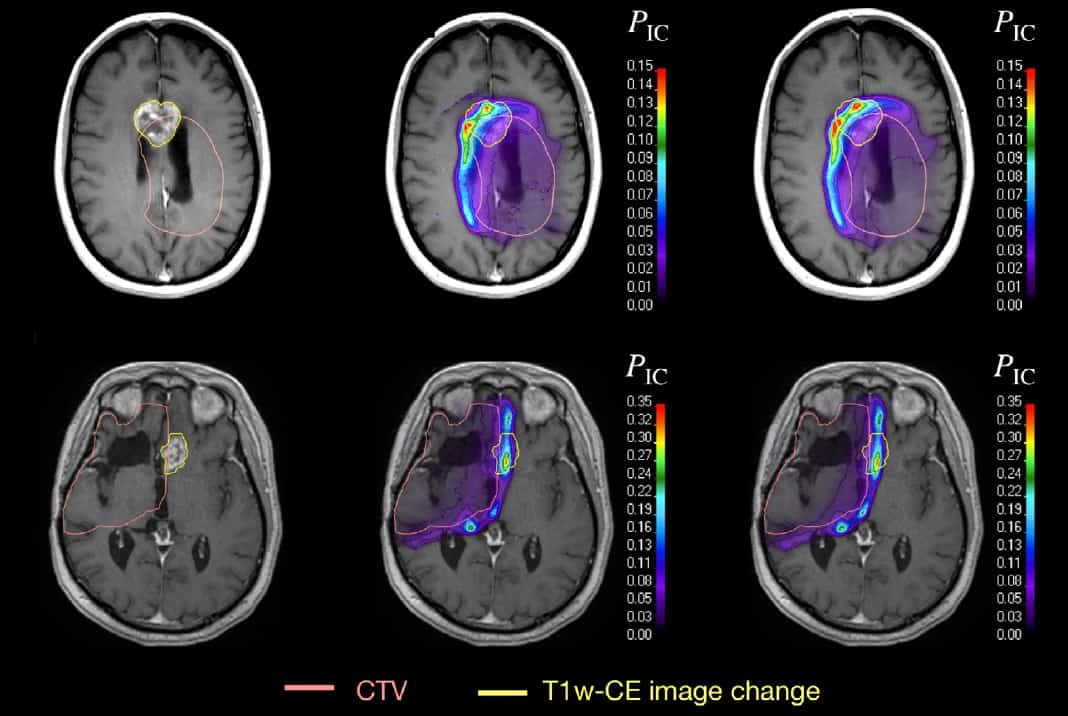
“We wanted to test whether the spatial distribution of late radiation-induced necrosis can be explained with dose alone or whether we need instead dose and LET, i.e., whether there is a variable dose response,” Lühr explains. “We consider this analysis as first clear evidence of a variable clinical RBE for a clinically relevant late endpoint.”
For the two multivariable models, the researchers also calculated the tolerance dose, TD50, at which 50% of brain tissue voxels experienced toxicity. Both models showed that TD50 decreased with increasing LET, indicating an increase in biological effectiveness.
The researchers conclude that their modelling framework revealed a spatially variable dose response in the brain and could predict the spatial distribution of image changes when using both dose and LET as predictors. The observed correlation of image changes with dose and increasing LET may indicate a variable clinical RBE different from 1.1, for glioma patients treated with proton therapy.
Proton therapy: not all RBE models are equal
“Due to the good performance of the models with dose and LET, we have the feeling that they are able to predict regions of elevated risk for radiation necrosis in the brain,” says Lühr. “However, to predict the absolute risk for individual patients we need to analyse a larger patient cohort.” He notes that the study has encouraged and enabled the team to now validate these findings in more patients, and that other proton centres plan to re-analyse their patient data in a similar way to look for similar effects.
“I believe that the clinical RBE is basically constant within the CTV, since only small LET variation occurs there. That is of high practical relevance in the clinic and allows for consistency in treatment dose prescription,” Lühr tells Physics World. “Outside of the CTV, however, the use of a variable clinical RBE for proton therapy planning might help reduce the risk of toxicity, especially for healthy tissue close to the CTV that receives high dose.”