Based on highly accurate optical interferometry and fundamental quantum calculations, researchers in the US are developing an improved definition of the SI unit for pressure that will consign the mercury manometer to history, explain Jay Hendricks, Jacob Ricker, Patrick Egan and Gregory Strouse

Many of us are unaware of the extent to which the international system of units (SI) touches our everyday lives, and this is especially true for pressure. Highly accurate, traceable measurements of pressure and vacuum conditions are vital in many manufacturing processes including that of semiconductor chips, where precise pressure measurements can improve control over features at the nanometre level. The routing of air traffic is another critical application: the minimum separation between aircraft as they approach busy airspace currently is about 600 m, but better pressure measurements could allow this distance to be cut in half – thereby reducing congestion and saving fuel.
Although pressure is one of the most widely measured units in everyday processes – and is key to vacuum science and technology – the standards that underpin it are very old. The SI unit for pressure, the pascal, is realized by mercury manometers that date back more than 300 years and we have now reached a point where the accuracy of such standards cannot be improved further. The same is true of temperature, the most widely measured unit, where the fundamental SI unit – the kelvin – is defined by the triple point of water.
A five-year project at the National Institute of Standards and Technology (NIST) in Gaithersburg, Maryland, US, aims to fundamentally change the way that the pascal is realized and disseminated. Based on ultra-precise measurements of the refractive index of gases using optical interferometry, it will raise pressure standards to the level of other SI units based on fundamental constants and also offer a brand new way to redefine the kelvin.
Mercury falling
The story of barometric pressure and early vacuum measurements is the story of mercury manometers, which were invented in 1643 by Italian physicist and mathematician Evangelista Torricelli. Some of the earliest barometric measurements were made by taking liquid in a glass–mercury manometer up a mountain and periodically observing the height of the mercury column along the way: the higher the altitude, the lower the mercury column height, indicating a lower barometric pressure. Named in Torricelli’s honour, the torr (equal to 133.322 Pa) is nominally equal to 1 mm of mercury-column height and the unit is still in common use today on many vacuum gauges.
Since Torricelli’s day, improvements in the mercury manometer have been incremental, based on resolving column heights with greater precision and accuracy. The lowest pressure uncertainties today come from a device called the ultrasonic interferometer manometer developed at NIST in 1975, which uses pulsed ultrasound to determine column heights in a 3 m-tall mercury liquid-column manometer to within 10 nm. But we have now reached the limit of such improvements. Furthermore, mercury’s status as a neurotoxin and environmental hazard has led to a ban on the purchase of mercury products. Government laboratories, national laboratories and corporations have therefore been forced to get rid of their mercury manometers, and as a result have seen their pressure-measurement capabilities downgraded. However, NIST still maintains its three mercury manometers as the national standard for the US – and will continue to do so until a high-accuracy, mercury-free alternative has been developed.
We have reached the limits of improvements based on mercury manometers
Since reference manometers are not portable (the NIST device is 3 m high and contains 250 kg of mercury), a parallel goal is to build a pressure standard that can deliver pressure measurements directly to the community. Several years ago, NIST researchers took an intermediate step towards this goal by developing a mercury-free “transfer standard package” (TSP) that enables high-quality measurements of NIST’s mercury manometers to be transferred to industry, academia and national standards laboratories around the world. This device allows reliable pressure measurements ranging from high vacuum (10–2 Pa) to atmospheric conditions (105 Pa), where improper use or incorrect calibration of gauges can cost time and money. But even though it provides a bloodline that links vacuum gauges directly to the SI system, the TSP still requires NIST to maintain and operate a 3 m-high mercury manometer. Our new project aims to replace not only the mercury manometer, but also the TSP, and do it at higher accuracy with an even more compact device.
Pressure by refractive index
In search of a better, mercury-free way to realize pressure – and one based on fundamental physics rather than a physical object – NIST has embarked on an entirely new optical pressure standard that links the pascal to quantum calculations of helium’s refractive index. Pressure and vacuum standards based on refractive index will significantly cut measurement uncertainties and improve accuracy in the aerospace, energy and advanced manufacturing sectors by between three- and 10-fold.
The refractive index of a gas depends on its density, which is a function of temperature and pressure. Quantum mechanics tells us the exact relationship between these variables, and calculations for atomic helium will allow us to relate pressure, temperature and refractive index with an accuracy significantly better than one part in 106. By measuring temperature and refractive index to high accuracy, we can determine the pressure and turn the device into a primary pressure standard based on the atomic properties of helium. Conversely, if pressure can be measured accurately using alternative means, then thermodynamic temperature can be determined by measuring the refractive index.
The key to making this approach worthwhile is to measure refractive index with much higher accuracy than has previously been achievable, which is possible using laser interferometry. When measuring a length using laser interferometry, the presence of a gas such as helium causes the distance to look a little bit longer than would be observed in vacuum. If we were able to compare two identical lengths in helium and in vacuum, the apparent difference would therefore allow us to determine the refractive index. The challenge facing NIST is that this difference must be measured with an accuracy of a few picometres – about 1% of a typical atomic diameter. Although picometre precision is relatively straightforward using a Fabry–Pérot interferometer, in which light bounces back and forth many times in an optical cavity, we face the challenge of generating identical displacements in two optical cavities (one in vacuum and one in helium) both with picometre accuracy.
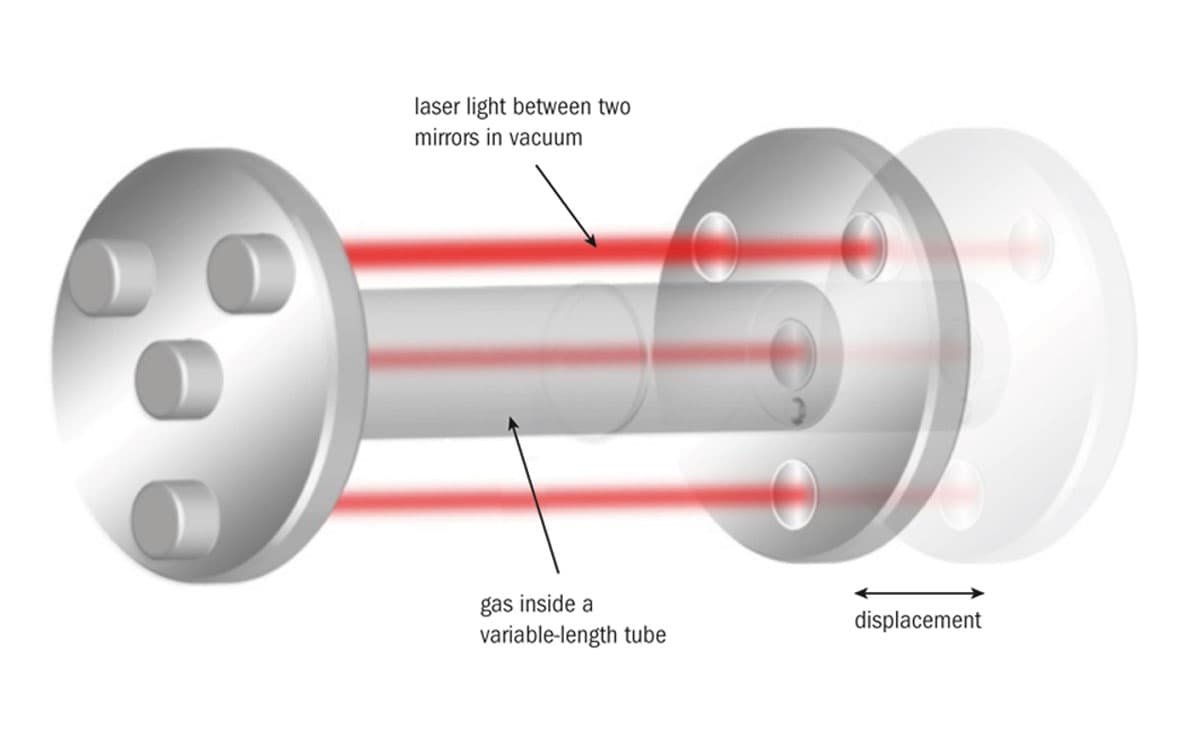
To this end, NIST is developing a variable-length optical cavity (VLOC) comprising four individual cavities: a central cavity in helium gas surrounded by three cavities in vacuum (see figure). The mirrors at each end of the apparatus are built on highly stable bases such that the displacement of one mirrored end-piece generates equal displacements in both the outer and inner interferometers. The reason for having three interferometers in vacuum is that it allows measurement and control of angular tilts as the end-piece is translated, which is necessary to avoid Abbe errors.
Building such a primary pressure standard at NIST is all well and good, but we also have to find a way to transfer its superior accuracy to locations outside the standards lab. We are therefore also developing a less complex fixed-length optical cavity (FLOC), which will offer enhanced sensitivity over the current TSP in a greatly simplified and more portable package. The transportable FLOC standard, a prototype of which is currently being built, will use nitrogen as a working medium because it has a higher refractivity than helium and is 100 times less sensitive to contaminants. Fixed-length cavities are attractive as pressure standards because they can be made from materials with small temporal instabilities, and will deliver an immediate improvement by a factor of three in pressure-measurement uncertainties over existing commercial technologies for vacuum pressures down to 1 Pa. NIST plans to employ the FLOC and VLOC as optical pressure and vacuum standards for pressures up to 360 kPa, which will allow us to replace all mercury-based pressure standards within the next 5–10 years.
Branching out
The five-year-long project is currently in its second year. The design phase has been completed, custom parts are currently being fabricated and NIST plans to have a fully working prototype by the summer of 2015. Over the next three years, we will complete the VLOC and refine the quantum-mechanical calculations of helium’s refractive index. This will also allow us to measure the refractive index of nitrogen to a level sufficient to make the FLOC a standalone pressure and vacuum standard. Finally, the optical standard will be compared with the existing NIST mercury manometers to close the final chapter on Torricelli’s mercury pressure standard.
Since the new optical pressure standard does not require the long central column that mercury manometers do, there is essentially no limit to how small the device can get. While the first generation of FLOCs will be 15 cm in length (which is already 20 times smaller than NIST’s mercury manometer), future versions may become still smaller and more compact to deliver better pressure measurements directly to the pressure and vacuum community. Even more exciting is that the unit for pressure will be quantum-based. Manometers have a direct link back to the SI through high-accuracy measurements of elemental mercury’s density that is traceable to the kilogram. However, the new standard will no longer be linked to the kilogram, which is an artefact-based standard, but based on the refractive index of helium and on fundamental constants that are the same every-where in the universe.
The same technology can be used to determine thermodynamic temperature with uncertainties below what has been previously achieved, providing a definition of the kelvin that is independent of water’s triple point. The NIST project also applies to dimensional metrology using laser interferometry, for which accuracy can be limited by uncertainty in the refractive index of air. With the FLOC cavity being open to the environment, it provides in situ measurements of the local refractive index and therefore allows real-time wavelength corrections to be made with a projected uncertainty at the level of three parts in 109 when measuring large distances, say on a factory floor.
NIST’s new mercury-free optical pressure standard will benefit science, industry and defence applications, as well as secondary-measurement services. Indeed, the FLOC and VLOC devices will ultimately eliminate mercury from all NIST primary pressure standards and at other labs and industrial facilities. We expect the optical pressure standard to become a commercial device used across academia and industry at a fraction of the cost of a commercial pressure standard, while being cheaper to maintain and having a larger pressure range and lower uncertainty.



