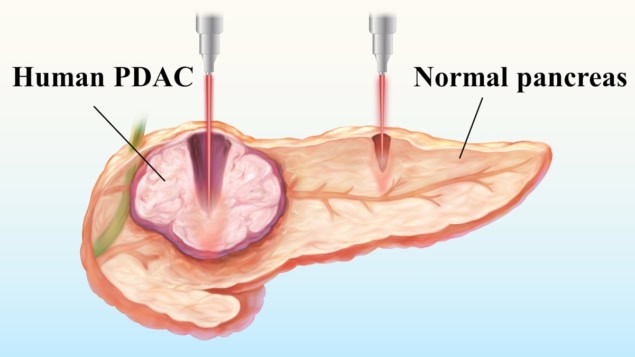
Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer, is an aggressive tumour with a poor prognosis. Surgery remains the only potential cure, but is feasible in just 10–15% of cases. A team headed up at Sichuan University in China has now developed a selective laser ablation technique designed to target PDAC while leaving healthy pancreatic tissue intact.
Thermal ablation techniques, such as radiofrequency, microwave or laser ablation, could provide a treatment option for patients with locally advanced PDAC, but existing methods risk damaging surrounding blood vessels and healthy pancreatic tissues. The new approach, described in Optica, uses the molecular fingerprint of pancreatic tumours to enable selective ablation.
The technique exploits the fact that PDAC tissue contains a large amount of collagen compared with healthy pancreatic tissue. Amide-I collagen fibres exhibit a strong absorption peak at 6.1 µm, thus the researchers surmised that tuning the treatment laser to this resonant wavelength could enable efficient tumour ablation with minimal collateral thermal damage. As such, they designed a femtosecond pulsed laser that can deliver 6.1 µm pulses with a power of more than 1 W.
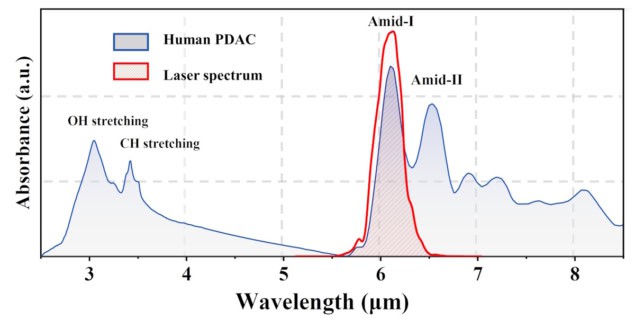
“We developed a mid-infrared femtosecond laser system for the selective tissue ablation experiment,” says team leader Houkun Liang. “The system is tunable in the wavelength range of 5 to 11 µm, aligning with various molecular fingerprint absorption peaks such as amide proteins, cholesteryl ester, hydroxyapatite and so on.”
Liang and colleagues first examined the ablation efficiency of three different laser wavelengths on two types of pancreatic cancer cells. Compared with non-resonant wavelengths of 1 and 3 µm, the collagen-resonant 6.1 µm laser was far more effective in killing pancreatic cancer cells, reducing cell viability to ranges of 0.27–0.32 and 0.37–0.38, at 0 and 24 h, respectively.
The team observed similar results in experiments on ectopic PDAC tumours cultured on the backs of mice. Irradiation at 6.1 µm led to five to 10 times deeper tumour ablation than seen for the non-resonant wavelengths (despite using a laser power of 5 W for 1 µm ablation and just 500 mW for 6.1 and 3 µm), indicating that 6.1 µm is the optimal wavelength for PDAC ablation surgery.
To validate the feasibility and safety of 6.1 µm laser irradiation, the team used the technique to treat PDAC tumours on live mice. Nine days after ablation, the tumour growth rate in treated mice was significantly suppressed, with an average tumour volume of 35.3 mm3. In contrast, tumour volume in a control group of untreated mice reached an average of 292.7 mm3, roughly eight times the size of the ablated tumours. No adverse symptoms were observed following the treatment.
Clinical potential
The researchers also used 6.1 µm laser irradiation to ablate pancreatic tissue samples (including normal tissue and PDAC) from 13 patients undergoing surgical resection. They used a laser power of 1 W and four scanning speeds (0.5, 1, 2 and 3 mm/s) with 10 ablation passes, examining 20 to 40 samples for each parameter.
At the slower scanning speeds, excessive energy accumulation resulted in comparable ablation depths. At speeds of 2 or 3 mm/s, however, the average ablation depths in PDAC samples were 2.30 and 2.57 times greater than in normal pancreatic tissue, respectively, demonstrating the sought-after selective ablation. At 3 mm/s, for example, the ablation depth in tumour was 1659.09±405.97 µm, compared with 702.5±298.32 µm in normal pancreas.
The findings show that by carefully controlling the laser power, scanning speed and number of passes, near-complete ablation of PDACs can be achieved, with minimal damage to surrounding healthy tissues.
To further investigate the clinical potential of this technique, the researchers developed an anti-resonant hollow-core fibre (AR-HCF) that can deliver high-power 6.1 µm laser pulses deep inside the human body. The fibre has a core diameter of approximately 113 µm and low bending losses at radii under 10 cm. The researchers used the AR-HCF to perform 6.1 µm laser ablation of PDAC and normal pancreas samples. The ablation depth in PDAC was greater than in normal pancreas, confirming the selective ablation properties.
“We are working together with a company to make a medical-grade fibre system to deliver the mid-infrared femtosecond laser. It consists of AR-HCF to transmit mid-infrared femtosecond pulses, a puncture needle and a fibre lens to focus the light and prevent liquid tissue getting into the fibre,” explains Liang. “We are also making efforts to integrate an imaging unit into the fibre delivery system, which will enable real-time monitoring and precise surgical guidance.”
Gold nanoparticles could improve radiotherapy of pancreatic cancer
Next, the researchers aim to further optimize the laser parameters and delivery systems to improve ablation efficiency and stability. They also plan to explore the applicability of selective laser ablation to other tumour types with distinct molecular signatures, and to conduct larger-scale animal studies to verify long-term safety and therapeutic outcomes.
“Before this technology can be used for clinical applications, highly comprehensive biological safety assessments are necessary,” Liang emphasizes. “Designing well-structured clinical trials to assess efficacy and risks, as well as navigating regulatory and ethical approvals, will be critical steps toward translation. There is a long way to go.”