With the International Year of the Periodic Table of Elements in full swing, John Campbell celebrates the immense contribution of Ernest Rutherford, who first split the atom 100 years ago

“You proved that atoms have balls.” So went a letter by a group of Russian physics students to Ernest Rutherford in 1929, in which they asked him to become an honorary president of their physics club. The Russian theoretical physicist George Gamow had to explain to a rather puzzled Rutherford that “atomic nucleus” in Russian has the same derivation as “cannonball” – and that the students had picked the wrong word from their Russian-English dictionary. Rutherford went off to write a letter of acceptance, laughing heartily.
Today, some eight decades after his death in 1937, the great New Zealander is best known for his discovery in 1911 of the atomic nucleus. Yet Rutherford had many other claims to fame, including one we celebrate this year. Exactly a century ago, in June 1919, Rutherford published four key papers in Philosophical Magazine, one of which was “IV. An anomalous effect in nitrogen”. It describes how he had become the first person to split the atom, induce a nuclear reaction and be a successful alchemist. Rutherford had shown, for the first time, that protons were constituents of nitrogen and that he had changed nitrogen into hydrogen.
Divide and conquer
The breakthrough can be traced back to when Rutherford was working in Canada in the early 1900s, where he showed that heavy atoms were not necessarily stable entities. Rutherford discovered that when he passed a beam of alpha particles through atmospheric air, or a thin slice of mica, the beam became fuzzy. The alpha particles were scattered by some two degrees, indicating that atoms must be “the seat of very intense electrical forces”. On arrival in Manchester in 1907, Rutherford set his assistant, Hans Geiger, to accurately measure how many were scattered through small angles.
Two years later, Rutherford then gave an undergraduate student, Ernest Marsden, a project to see if alpha particles were scattered back from a block of metal. Everyone knew that happened for beta particles (electrons), but no one expected the heavier alphas to do so too (Rutherford had given the alphas and betas their names in 1898). Marsden quickly showed that back-scattering occurred for alpha particles even when the block of metal was replaced with the same thin gold foils that were used in Geiger’s small-angle transmission experiments. It was to explain this effect that Rutherford in 1911 famously produced his nuclear model of the atom.
With heavier nuclei, a head-on alpha particle scattered before it got anywhere near the nucleus. But Rutherford thought that for light atoms, the alpha particles would get much nearer, possibly even colliding. In 1913 Rutherford therefore asked Marsden to fire alpha particles at light nuclei such as hydrogen. Classical physics showed that, in a head-on collision, the hydrogen ion (H+) would recoil at a speed 1.6 times that of an alpha and thus travel in air some four times farther. Rutherford and Marsden then duly observed the weak flashes of recoiling H+ ions on their scintillation screens.
Unfortunately, the collaboration effectively ended when Marsden moved to New Zealand in 1915 and Rutherford was absorbed in work for the First World War (see February 2016 pp32–36). When Rutherford returned to his research in 1917, he wrote to Niels Bohr in December saying the he was “trying to break up the atom”, which he urged Bohr to regard as “private”. Rutherford studied alpha impacts on many light gases and light atoms in solid form, such as hydrogen in paraffin wax. Yet when he substituted dry nitrogen for dry air, he found an anomaly: more H+ ions were produced than expected.
“We must conclude that the nitrogen atom is disintegrated under the intense forces developed in a close collision with a swift alpha particle, and that the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus,” he stated. “Considering the enormous intensity of the forces brought into play, it is not so much a matter of surprise that the nitrogen atom should suffer disintegration as that the α particle itself escapes disruption into its constituents”. And, he added, “if α particles – or similar projectiles – of still greater energy were available for experiment, we might expect to break down the nucleus structure of many lighter atoms”.
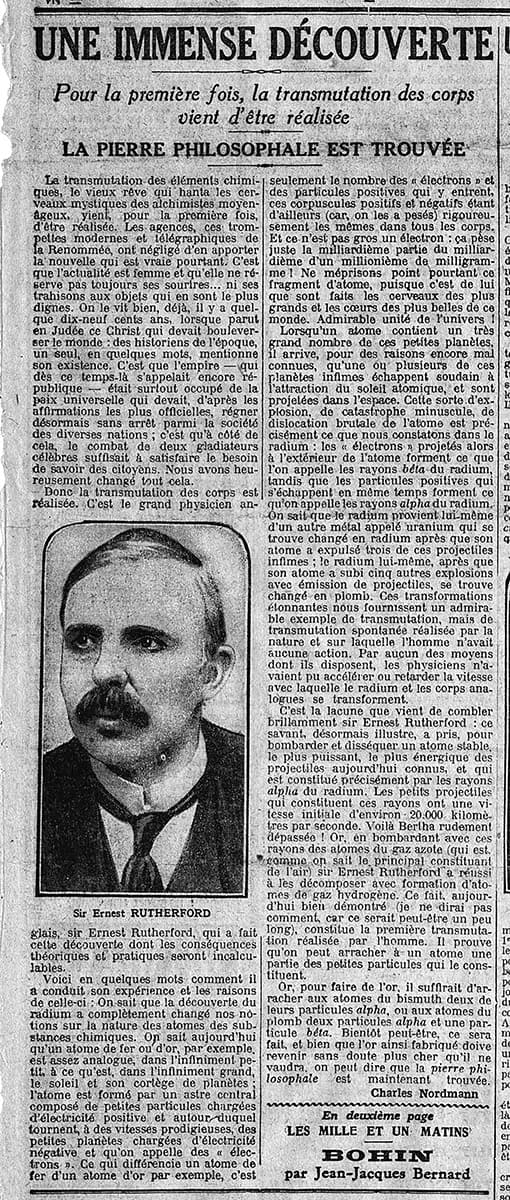
Rutherford submitted his four papers in April 1919 before leaving Manchester to succeed JJ Thomson as head of the Cavendish Laboratory in Cambridge. Paper “IV” initially did not create much public interest in Britain. That only occured when Charles Nordmann – a French astronomer and popularizer of science – visited Manchester in 1919 and wrote a front-page article for the Paris newspaper Le Matin on 8 December 1919 headlined “Une immense découverte” (“A tremendous discovery”), which was picked up by the Press Association. And when Rutherford delivered the Bakerian Lecture in 1920 at the Royal Society he predicted that the neutron must exist to account for isotopes and then began to construct atoms from their more fundamental building blocks.
With the advent of nuclear reactors and then high-energy particle accelerators, the 92-element periodic table of Rutherford’s day has now increased by 26 and includes an element named in his honour: Rutherfordium (Rf104). Indeed, the periodic table is expected to grow further in which each step requires adding one or more protons and neutrons – just as Rutherford suggested in 1920. As we celebrate the International Year of the Periodic Table of Elements in 2019, let us never forget the contribution of the man who not only pioneered our understanding of atoms – but also discovered that they could be split.