
Researchers from Syracuse University have achieved significant progress towards the engineering of large-scale bone tissue scaffolds (Biofabrication 10 035013).
Stephen Sawyer and colleagues have designed, built and tested a scalable platform for the structured growth of bone mineral using only a commercially available 3D printer and inexpensive materials. The design surpassed previous difficulties associated with the supply of oxygen to bone growing cells. Traditional designs relied on oxygen diffusion through the cell containing structure, which had, until now, limited the size of bone structures that could be built.
First, the researchers 3D printed a 9 × 6 × 3 mm heat-resistant frame together with a 1 mm wide sacrificial polyvinyl alcohol channel spanning the frame’s interior. This channel was key to the system design, as it can be removed in situ. This facilitated the perfusion of essential nutrients to the cells responsible for bone growth. Osteogenic, or bone-growing, media was then continually supplied through pressure driven infusion into the device. The frame was fitted into a machined polycarbonate housing that provided watertight sealing.
Sawyer and the team then encapsulated human osteosarcoma cells, which are essential in the early stages of bone growth, inside a gelatin methacrylate hydrogel. They pipetted the cell-laden gel into the device and hardened it by exposure to ultraviolet radiation, with negligible consequence for the cells inside the gel. Ingeniously, the 3D printed polyvinyl alcohol channels were then removed through the infusion of warmed cell media, which dissolves the sacrificial polyvinyl alcohol.
The researchers placed the entire device, including the syringe pump, inside an incubator to promote the development of bone mineral around the periphery of the channel. They followed the bone mineral development through a combination of fluorescence staining and micro-scale X-ray imaging.
Over the course of one month, nutrients were perfused through the device via a syringe pump. The perfused samples showed directed bone mineralization around the periphery of the channel. On the other hand, identical samples that were not perfused showed unstructured mineralization. Perfusion of nutrients proved essential to cell survival, with static samples exhibiting low cell viability after just two weeks of the one-month observation.
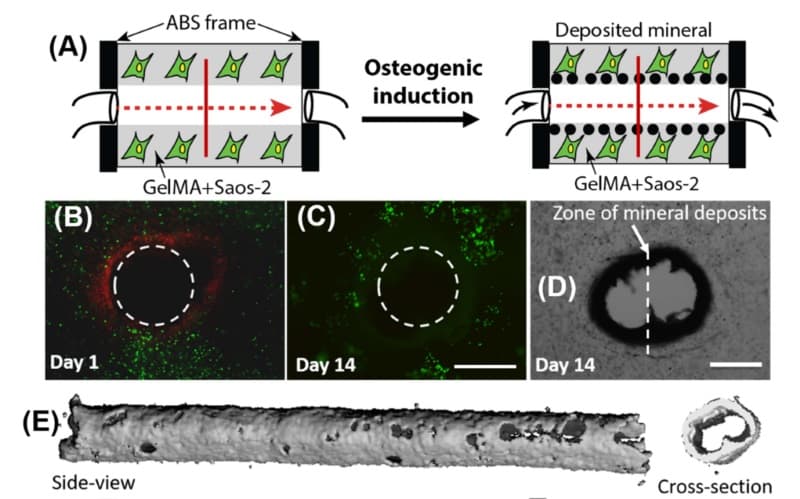
Moreover, the researchers demonstrated the easy scalability of the system by building a 3D array of channels within a single device. They used COMSOL modelling to identify the optimum spacing between channels based on the diffusion of oxygen through mineral clad channels and the gel matrix. Each channel showed promising bone mineral development after four weeks.
This work demonstrates the viability of user defined 3D printed channels together with commonly available sacrificial polymers and hydrogels in tissue engineering, and holds great promise for next-generation bone replacements.



