It is the hardest everyday material on Earth, so why does diamond glisten when rubbed against another diamond? Now, the ancient but mysterious process of diamond grinding may have been explained by physicists in Germany, who have created a model for explaining the frictional interactions at the molecular level.
For centuries precious-stone merchants have polished diamonds by grinding them with cast-iron wheels embedded with coarse diamond fragments. It is not clear why this procedure is so effective at cleaning diamonds, but experience suggests that it works far better when the diamond is fixed at certain angles to the wheel than others.
This directional dependence of diamond grinding has now been investigated by Lars Pastewka at the Fraunhofer Institute for Mechanics of Materials who set out to investigate the phenomenon. Working with colleagues at several other institutes across Germany, he has developed a quantum mechanical model to study the atomic interactions in “diamond-like” carbon films, which are often used in industry to reduce friction in machinery.
Diamond in the rough
But when the researchers applied their model to diamond itself, they were surprised to find that it accurately predicted the experimental wear rates for this material – even though the exact wear mechanism has so far remained poorly understood. “At this point we became very excited about this work and analysed our simulations in much more detail to uncover the details of the process,” Pastewka told physicsworld.com.
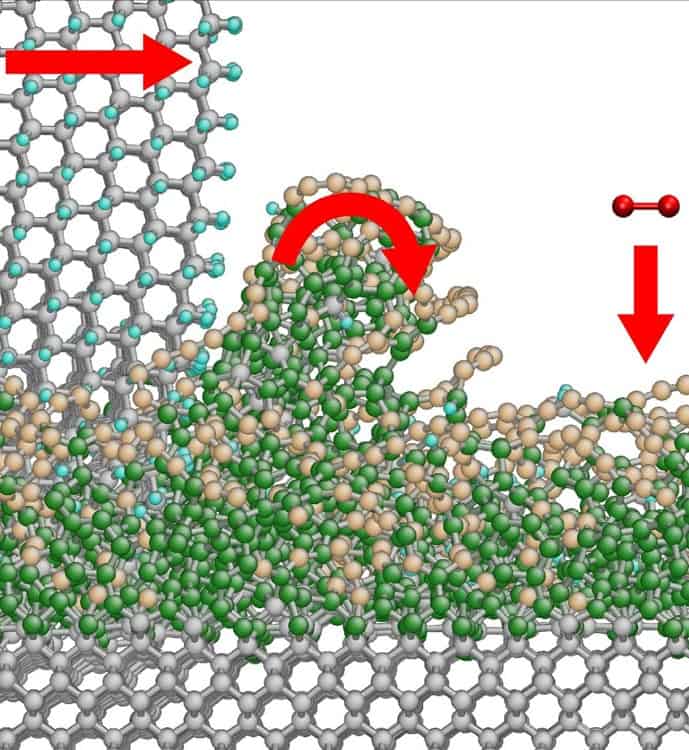
Pastewka’s team set about simulating diamond grinding using 70 computer processors running for a year, and discovered that during the grinding the diamond surfaces were being transformed into soft, amorphous layers. These thin films can then be easily removed by either chipping them away, or through carbon molecules bonding with oxygen in the atmosphere, leaving behind clean diamond surfaces.
This creation of the amorphous film occurs because of existing imperfections at the diamond surface, including the build-up of dirt over time. As a diamond atom slides over the surface it repeatedly pulls at the diamond crystal’s atoms, and sometimes removes an atom from the crystal surface, which becomes part of the amorphous layer.
Like a stack of paper clips
“Imagine you have a stack of paper clips neatly arranged on your desk,” explains Pastewka. “Now you take a magnet and move that over these clips at a certain height. You cannot keep the height ideally constant, so if the height is right you will pull some paper clips to your magnet and others will remain on the desk.”
Changfeng Chen, a materials scientist at the University of Nevada in the US is impressed by the research and its potential to boost industrial processes. “This research is of particular significance in nanotechnology where the orientations of nanoscale crystallites can be well defined and controlled,” he says. “The predicted orientation-dependent anisotropic amorphization wear mechanism may open doors to a new level of material processing, ranging from better designer jewellery to superior high-tech device components.”
To develop the work, however, Pastewka’s team intends to further investigate diamond’s surface chemistry, and is currently writing a paper on the oxidation of the amorphous layer.
This research is described in a research paper in Nature Materials.