One of the rarest metals in the universe, metallic hydrogen could solve many energy problems – but has it finally been isolated in the lab? Jon Cartwright tries to sort out claim from counter-claim

Scientists aren’t immune to the allure of rare metals. Isaac Newton’s interest in alchemy is well documented: when the natural philosopher wasn’t laying the groundwork for much of modern physics he was often secretively, obsessively, attempting to turn lead into gold. These days, physicists are hoping to turn a humble element into something yet more precious.
Hydrogen is the lightest of all atoms, not to mention the most abundant, accounting for three-quarters of all the universe’s normal matter. As we commonly know it, hydrogen is a molecular gas – colourless, odourless, mostly harmless and, some might say, rather dull. But under extreme pressure, hydrogen will supposedly turn into one of the rarest metals in the universe, one that is naturally non-existent here on Earth and perhaps only present in the underworlds of gas giants, such as Jupiter. The metal is coveted not just for its rarity, but also because it could turn out to be a stable room-temperature superconductor, and therefore go a great way to solving the world’s energy problems.
For over a century physicists have sought metallic hydrogen. Within the past year, however, physicists Isaac Silvera and Ranga Dias (pictured above) at Harvard University in Massachusetts, US, claim they have finally made it, by squeezing hydrogen inside a diamond anvil cell to pressures of nearly five million atmospheres. Is it for real? “If it is true, it is a great achievement, fulfilling a long search for the atomic phase of hydrogen,” says David Ceperley, a theorist at the University of Illinois Urbana–Champaign in the US. “However, there is deep scepticism in the community about their experiment.”
Early searches
Subjecting hydrogen to extreme conditions is certainly nothing new. It was on the cusp of the 20th century that the British scientist James Dewar first cooled hydrogen until it turned solid, at temperatures of less than 14 K. Around this time, chemists were also mulling over the element’s position in the periodic table. Situated at the top of a column of alkali metals, hydrogen ought to form a metal itself, given the right conditions. Those conditions were first calculated in 1935 by the Hungarian-born physicist Eugene Wigner and the US physicist Hillard Bell Huntington, who predicted that at temperatures close to absolute zero, and at pressures of about 250,000 atmospheres (25 GPa), hydrogen atoms ought to become so closely packed that their electron clouds overlap, as in a metal.
Wigner and Huntington had fired the starting pistol in the search for metallic hydrogen, but their prediction was optimistic. With the invention of the diamond anvil cell at the National Bureau of Standards (now the National Institute of Standards and Technology, or NIST) in the late 1950s, high pressures suddenly became routinely accessible; and by the 1970s, they were topping 100 GPa – yet for hydrogen, there was no sign of a metal transition.
Still, hydrogen’s phase diagram could be mapped with ever greater clarity (figure 1). The element is always a solid at high pressure and at moderate temperatures, but takes on distinct phases depending on just how high the pressure is. Phase I is a close-packed structure in which the molecular angles are disordered. Phase II is a similar structure but with some degree of orientational order. Phase III is believed to be a structure in which the strength of the hydrogen–hydrogen bonds turns so weak that the hydrogen can be considered partly atomic, not molecular. In phase IV, some evidence suggests a portion of the hydrogen molecules form planar, graphene-esque sheets. Beyond those phases? Metallic hydrogen – possibly.

While all this phase-mapping has been taking place, theorists have been coming up with rewards for isolating the elusive metal. In the late 1960s, physicist Neil Ashcroft at Cornell University in New York, US, predicted that solid metallic hydrogen could be a high-temperature superconductor; and in the past decade, other theorists have suggested that the critical temperature for such a superconductor could be above room temperature, unlike any other known to date.
Another use that Silvera and others have recently speculated is that the substance could serve as a rocket fuel. According to their calculations, solid metallic hydrogen’s specific impulse – the standard measure of a propellant – would be more than three and a half times greater than the mix of liquid hydrogen and oxygen traditionally used to fuel rockets, allowing far more ambitious space travel. Granted, creating the conditions for solid metallic hydrogen in a fuel tank would not be easy, but calculations in the early 1970s by physicists at the Soviet Union’s I V Kurchatov Institute of Atomic Energy in Moscow suggested that solid metallic hydrogen could actually be metastable at room temperature and pressure: once created, it would stay a metal even when the pressure is released.
Not that such applications have driven Silvera in his quest for the metal. “I’ve always been interested in very challenging problems,” he says. “And in the high-pressure research community, metallic hydrogen was always considered to be one of the greatest challenges that existed.” He began his search in the 1970s while a professor at the University of Amsterdam in the Netherlands, and though other topics have occasionally diverted him – in 1979, for example, he and his Amsterdam colleague Jook Walraven made hydrogen into the first quantum gas, the precursor to a Bose–Einstein condensate – metallic hydrogen has always been at the forefront of his mind.
For most scientists in the field there is little doubt that the state exists. Indeed, it is believed that hydrogen in the highly compressed cores of the larger gas giants, such as Jupiter, must be metallic – albeit probably liquid rather than solid in form – in order to generate their observed magnetic fields. “Everyone believed that if you got hydrogen to a high enough density, which means a high enough pressure,” says Silvera, “that it would become metallic.” Quite where the transition lay, however, no-one was sure, and ramping up the pressure to find out has not been easy.
Diamond anvil cells consist of a pair of diamonds that have had their points polished down to a small flat tip known as a culet. The hydrogen sample is compressed between the two culets within a containing gasket, having been loaded in gas or liquid form. But if you turn the pressure up too high, the diamonds themselves are liable to fail.
About a decade ago, however, several experimental groups found ways of boosting the pressures of hydrogen within diamond anvil cells from the long-held maximum of about 200 GPa to nearly 400 GPa – higher than the centre of the Earth – but this appeared to be another plateau. Unfortunately, attaining these pressures is an art as much as a science, as it is not always clear what causes the diamonds to break.
Lab elation
In recent years, Silvera and Dias, his postgraduate student, have been developing ways to deliver yet higher pressures. Figuring that defects are one of the causes of premature diamond failure, they etch away the surface of their synthetic diamonds with an ion gun, to remove any layers of carbon atoms that have been accidentally gouged during the culet-polishing process. They have also got into the habit of coating their diamonds with alumina – otherwise known as sapphire when it is a gemstone – which should prevent any hydrogen diffusing into and embrittling them. Finally, they avoid any continuous illumination with laser light to study the experiments in progress, as it is believed to be a trigger for failure. “That turned out to be important,” Silvera recalls.
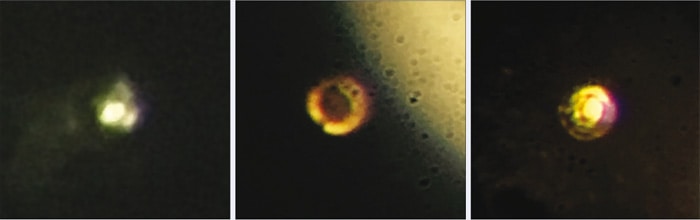
So it was that in October last year, working in his office, Silvera was interrupted by an excited Dias at his door, and the pair rushed back to the lab where the postdoc had been stepping up the pressure in their latest cell, at temperatures lower than 83 K. Seen through an optical microscope, the hydrogen sample was no longer matt black, but had a metallic lustre. Both Silvera and Dias knew what this meant and, having nothing better to hand, took pictures of the sample with an iPhone. “People often asked me, how are you going to know if you’ve got metallic hydrogen?” says Silvera. “And I would say: by all the yelling and screaming coming out of the laboratory. My whole group was very excited.”
According to Silvera, there are two ways to demonstrate that a substance is metallic. The first is to show that its DC conductivity stays finite as its temperature is brought to absolute zero – a measurement that requires four electrical leads to remain in contact with the microscopic sample inside the cell as, with rising pressure, its volume diminishes 15-fold. Having no apparatus to do that, the researchers instead opted for the second method: AC conductivity, which manifests as reflectance and can be measured via the CMOS camera on a suitable microscope. Fitting these measurements at several wavelengths to the well-established Drude model of electrical conduction, the researchers found that there was the same density of electron carriers as the density of atoms in the system, a hallmark of a metal (Science 10.1126/science.aal1579).
The celebration was perhaps shorter lived than Silvera and Dias would have liked. Minus a laser, they were only able to roughly estimate the pressure on their hydrogen indirectly via strain gauges on the cell: 495 GPa, give or take 5–10%. Others, they knew, would want a more reliable reading, particularly given the fact that it was substantially greater than most groups report. Tentatively, then, the Harvard pair set up a weak, half-milliwatt laser to take a reading. “We turned the laser on: one of the diamonds broke,” says Silvera. “Catastrophically – there was nothing left but powder, like flour.” The precious metal had apparently slipped away.
Sceptical response
Many other experimentalists, however, believe it was not there in the first place. Eugene Gregoryanz of the University of Edinburgh in the UK has been one of the most vocal critics: on a scale of 1 to 10, where 10 is the most sceptical, he places himself at 11. He says Silvera and Dias’ paper, in which they report their findings, is inexcusably short on data, and believes that the hydrogen in Silvera and Dias’s cell might well have escaped before the highest pressures were reached – if indeed such pressures were reached, as he doubts the tips of the diamonds used were small enough to generate them. The apparent lustre in the photos is, he says, the rhenium gasket itself after the hydrogen has left – a phenomenon he has witnessed in his own experiments. “The so-called paper is so bad, there’s not a single point you can discuss,” he adds.
Aside from Gregoryanz and colleagues’, there are three other comments on arXiv by other experimental groups in the field criticizing various aspects of Silvera and Dias’ report. The comments claim variously that the Harvard group’s optical measurements are not definitive proof of a metallic transition; that the hydrogen observed may be contaminated by leaked rhenium; and that the pressure estimations are primitive. In a written response, Silvera and Dias reject each of the claims in turn. “I haven’t yet heard a good physical objection to our experiment,” Silvera says, now. “Basically [the sceptics] are competitors, and some of them are really good scientists, and have been trying to make metallic hydrogen for a number of years. They are not so happy to see someone else do it.”
“Look,” he continues. “If you had made metallic hydrogen, would you wait another year to show the world that you can make it? Or would you publish it straight away, as soon as you had made it the first time? We decided to publish right away, and continue with the studies.”
From a historical point of view, Silvera’s decision might be seen as a gamble. His and Dias’ claim is not the first for metallic hydrogen: in 2011, Mikhail Eremets and Ivan Troyan of the Max-Planck Institute for Chemistry in Mainz, Germany, believed they had isolated the state at a pressure of 220 GPa (Nature Mater. 10.1038/nmat3175). Then, as now, others were doubtful, and the following year Eremets reportedly admitted that he wished their paper had not made such a definitive announcement. (Eremets did not respond to questions about the claim when contacted by Physics World.) Even as this issue went to press, a paper by Russell Hemley at the George Washington University in Washington DC, US, and colleagues, accepted for publication in the journal Physical Review Letters, cautiously reports evidence for metallic hydrogen at modest temperatures and at a pressure near 300 GPa. Ceperley, Gregoryanz and indeed Silvera too are unconvinced.
Repetition is key
The one point on which everyone agrees is that the reproduction of results is the only way forward. “To my knowledge the single experiment reported by Dias and Silvera has yet to be reproduced,” says Bill Nellis, another Harvard experimentalist. He believes it is conceivable that, even at the low temperatures of Silvera and Dias’ experiment, there are chemical reactions between the hydrogen in the sample and the carbon in the diamond anvil cell. “In this case the observed reflectance might be caused by the formation of a new hydrogen–carbon phase, rather than the closure of an electronic band gap in monatomic hydrogen,” he explains.
Silvera accepts the need for more results. But while far from Newton’s alchemy, the experiment is still not as straightforward to reproduce as it might at first seem, even with a recipe that has been honed over years of experience. In one recent attempt the diamonds, which were not of optimum size, achieved a pressure just under 400 GPa before failing, Silvera says. Meanwhile, another batch of 10 diamonds turned black during a usual annealing process – suggesting air had leaked into the annealing chamber – and had to be discarded. “These diamonds cost $1000–1500 each,” Silvera points out. At the time of writing this article, another batch of diamonds had arrived and were being prepared. If those work, the results could be available in a matter of weeks; if not, it will be several months.
Needless to say, Silvera is as eager to have them as anyone else. “I’m running the experiment whenever I’m not on the telephone,” he says.