Researchers in Japan say that they have made 2D honeycomb crystals of silicon that resemble the carbon-based material graphene. This is the second potential sighting of the material dubbed “silicene”; the other was reported in April by an independent group in Europe. The Japanese research suggests it may be relatively easy to alter the structure of silicene by changing the substrate on which it is grown – which could allow different versions of silicene to be produced with a range of useful electronic properties. However, not all scientists agree that this latest material is actually silicene.
Graphene is a honeycomb lattice of carbon just one atom thick and since its discovery in 2004 the material has proved to have a myriad of interesting and potentially useful properties. As well as being the material of choice in the electronics industry, silicon lies directly below carbon in the periodic table. The latter suggests that if silicon atoms were deposited in a layer on an appropriate surface, they could arrange themselves in a honeycomb lattice to make silicene – which would have properties similar to graphene.
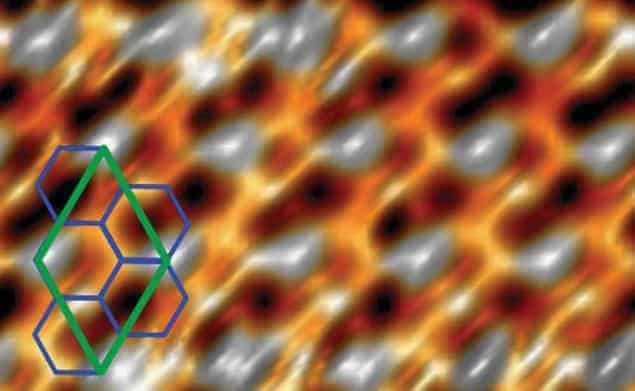
Silicon on silver
This was first done by a team of researchers in Italy, Germany and France that included Paola de Padova of the Consiglio Nazionale delle Ricerche-ISM in Rome. De Padova and colleagues deposited silicon on a silver crystal and found the film to have the structural and electronic properties expected of silicene.
Now, a team in Japan led by Yukiko Yamada-Takamura of the Japan Advanced Institute of Science and Technology in Ishikawa say that it has created a modified form of silicene on a substrate of zirconium diboride. The crucial difference to the previous work is that while silver has a very similar lattice constant to that expected of silicene, the lattice constant of zirconium diboride is quite different. As a result, silicene grown on silver adopts a flat, graphene-like atomic configuration and has very similar properties to the carbon-based material. Silicene on zirconium diboride, on the other hand, has a distorted lattice structure.
It is this ability to distort the silicene lattice that appeals to Yamada-Takamura and colleagues. This is different to the structure of graphene, which is very stable. This stability is a problem because altering graphene’s structure could provide researchers with a way of creating an electronic band gap in the material – a development that would allow graphene to be used in a wide range of electronic applications.
Flexible on an atomic scale
The team describes silicene as being very “flexible” and says that it should be possible to manipulate its structure in ways that are virtually impossible with graphene. “You can roll a sheet of graphene or something like that,” explains Yamada-Takamura, “but what we mean by flexible is that silicene can be atomitistically flexible, so the atoms can be displaced out of the plane.” This buckling out of the plane leads to very different electronic properties and by depositing silicon on a variety of different substrates, the researchers believe it may be possible to produce a whole family of silicenes that offer a range of different electronic properties.
De Padova is impressed by this latest work, but is cautious about describing the new material as silicene. “I think the paper is interesting because there is, in principle, evidence for the growth of silicene on, for example, zirconium diboride and not only on silver.” She is reluctant, however, to endorse the researchers’ conclusion that their material is a modified form of silicene. She also has doubts about whether the work shows that the properties of silicene can be modified by epitaxial strain. She suggests instead that the substance produced on the surface of the zirconium diboride may not be silicene at all. Yamada-Takamura responded “that depends how you define silicene”.
The research is published in Physical Review Letters.



