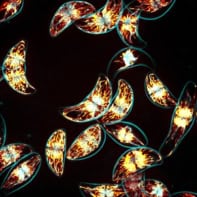
A new “sneeze simulator” could help scientists understand how respiratory illnesses such as COVID-19 and influenza spread. Built by researchers at the Universitat Rovira i Virgili (URV) in Spain, the simulator is a three-dimensional model that incorporates a representation of the nasal cavity as well as other parts of the human upper respiratory tract. According to the researchers, it should help scientists to improve predictive models for respiratory disease transmission in indoor environments, and could even inform the design of masks and ventilation systems that mitigate the effects of exposure to pathogens.
For many respiratory illnesses, pathogen-laden aerosols expelled when an infected person coughs, sneezes or even breathes are important ways of spreading disease. Our understanding of how these aerosols disperse has advanced in recent years, mainly through studies carried out during and after the COVID-19 pandemic. Some of these studies deployed techniques such as spirometry and particle imaging to characterize the distributions of particle sizes and airflow when we cough and sneeze. Others developed theoretical models that predict how clouds of particles will evolve after they are ejected and how droplet sizes change as a function of atmospheric humidity and composition.
To build on this work, the UVR researchers sought to understand how the shape of the nasal cavity affects these processes. They argue that neglecting this factor leads to an incomplete understanding of airflow dynamics and particle dispersion patterns, which in turn affects the accuracy of transmission modelling. As evidence, they point out that studies focused on sneezing (which occurs via the nose) and coughing (which occurs primarily via the mouth) detected differences in how far droplets travelled, the amount of time they stayed in the air and their pathogen-carrying potential – all parameters that feed into transmission models. The nasal cavity also affects the shape of the particle cloud ejected, which has previously been found to influence how pathogens spread.
The challenge they face is that the anatomy of the naval cavity varies greatly from person to person, making it difficult to model. However, the UVR researchers say that their new simulator, which is based on realistic 3D printed models of the upper respiratory tract and nasal cavity, overcomes this limitation, precisely reproducing the way particles are produced when people cough and sneeze.
Reproducing human coughs and sneezes
One of the features that allows the simulator to do this is a variable nostril opening. This enables the researchers to control air flow through the nasal cavity, and thus to replicate different sneeze intensities. The simulator also controls the strength of exhalations, meaning that the team could investigate how this and the size of nasal airways affects aerosol cloud dispersion.
During their experiments, which are detailed in Physics of Fluids, the UVR researchers used high-speed cameras and a laser beam to observe how particles disperse following a sneeze. They studied three airflow rates typical of coughs and sneezes and monitored what happened with and without nasal cavity flow. Based on these measurements, they used a well-established model to predict the range of the aerosol cloud produced.

“We found that nasal exhalation disperses aerosols more vertically and less horizontally, unlike mouth exhalation, which projects them toward nearby individuals,” explains team member Salvatore Cito. “While this reduces direct transmission, the weaker, more dispersed plume allows particles to remain suspended longer and become more uniformly distributed, increasing overall exposure risk.”
These findings have several applications, Cito says. For one, the insights gained could be used to improve models used in epidemiology and indoor air quality management.
Nanoparticles demonstrate new and unexpected mechanism of coronavirus disinfection
“Understanding how nasal exhalation influences aerosol dispersion can also inform the design of ventilation systems in public spaces, such as hospitals, classrooms and transportation systems to minimize airborne transmission risks,” he tells Physics World.
The results also suggest that protective measures such as masks should be designed to block both nasal and oral exhalations, he says, adding that full-face coverage is especially important in high-risk settings.
The researchers’ next goal is to study the impact of environmental factors such as humidity and temperature on aerosol dispersion. Until now, such experiments have only been carried out under controlled isothermal conditions, which does not reflect real-world situations. “We also plan to integrate our experimental findings with computational fluid dynamics simulations to further refine protective models for respiratory aerosol dispersion,” Cito reveals.