Monitoring tumour progression during a course of radiation therapy can help determine whether a treatment is working or not. Such tracking is mostly anatomy-based, using weekly CT scans, for example, to measure tumour size. This approach, however, can fail to detect subtle changes at the cellular level – a task that calls for functional imaging.
Functional imaging modalities can characterize responses of the tumour microenvironment. But common techniques, such as PET or diffusion-weighted MRI, require a separate scheduled exam. Researchers from Dartmouth College’s Thayer School of Engineering have now proposed a way to image the tumour microenvironment during radiotherapy without interrupting the clinical workflow, using Cherenkov-excited luminescence imaging (CELI). They achieve this by employing CELI to track the spread of a phosphorescent tattoo ink (Phys. Med. Biol. 10.1088/1361-6560/ab7d16)
CELI works by using the Cherenkov light generated as the treatment beam travels through tissue to excite a luminescent agent – in this case, a UV-sensitive tattoo dye injected into the tumour. The researchers propose that as tumour cells break down in response to irradiation, the dye will spread. This diffusion can then be measured during radiotherapy by using a camera to image the emitted phosphorescence signals.
“In the last few years, our lab has done a great deal of radiotherapy research focused on imaging the radiation beam delivered to the patient in real time using time-gated Cherenkov imaging,” says first author Jennifer Soter, a PhD student in Brian Pogue’s research group. “We were prompted to expand on this technology further and explore additional applications in cancer treatment. In the case of tumour progression in radiotherapy, there’s really no feasible method that enables daily imaging of tumours clinically.”
In vivo investigations
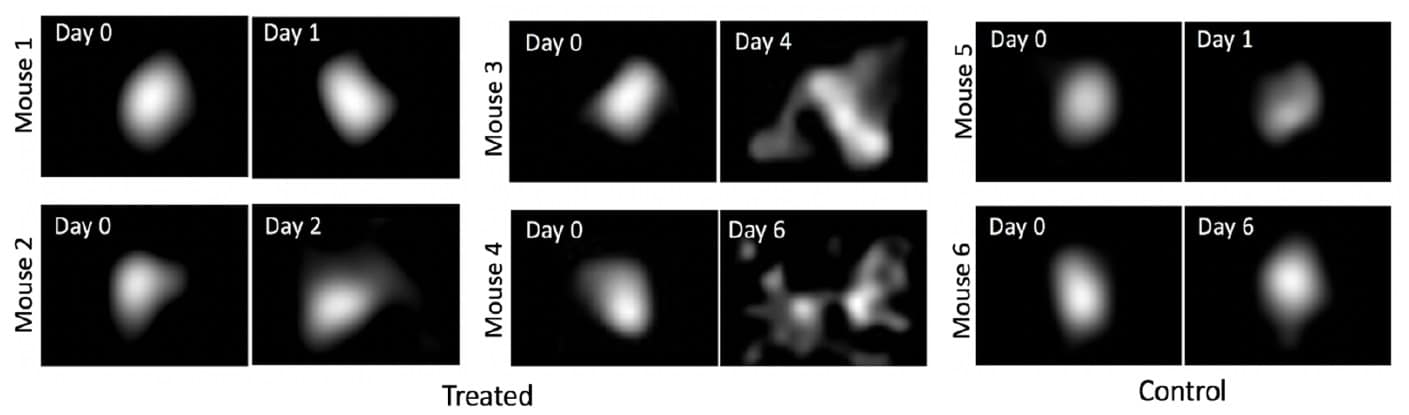
Soter and colleagues used a mouse model to evaluate whether CELI can directly track subtle changes in the tumour microenvironmental in response to radiation therapy. On day zero, they injected tattoo ink into the centre of tumours in 20 mice. They then delivered a 1.4 Gy treatment fraction to all mice, using 6 MV X-ray beams from a clinical linac, and performed a baseline CELI session to measure the initial spread of the ink.
The researchers used an intensified CMOS camera to detect visible phosphorescence from the ink. The camera was time-gated with the linac pulses, such that it only recorded the delayed phosphorescence signals emitted between each radiation pulse. Immediately after the first CELI session, they delivered an additional 12 Gy dose to 15 mice, while nine untreated controls received no additional radiation. One to six days later, they delivered a second 1.4 Gy to each mouse and performed the final CELI session
By comparing images acquired immediately after injection with the final diffusive ink spread, the researchers could determine the tumour response. “Cell death via apoptosis and necrosis can lead to significant sections of the tumour decreasing, resulting in tissue clearance, which is well known from diffusion MRI,” explains Soter. “The ink is a simple label that also diffuses from within the tumour as the pressure decreases.”
In the control group, ink distributions remained constant after four days, with less than 2% diffusive spread. In treated mice, on the other hand, the ink spread reached almost 200% by day six. From two days post-injection, the team could see a significant difference in diffusive spread values between treated mice and control mice.
Following the final CELI session, the researchers euthanized the mice and imaged tumours using hyperspectral cryo-fluorescence imaging to quantify radiation-induced necrosis. Different regions of tumour – the non-perfusing necrotic core, the viable tissue and the dye – exhibited clearly different reflectance spectra.
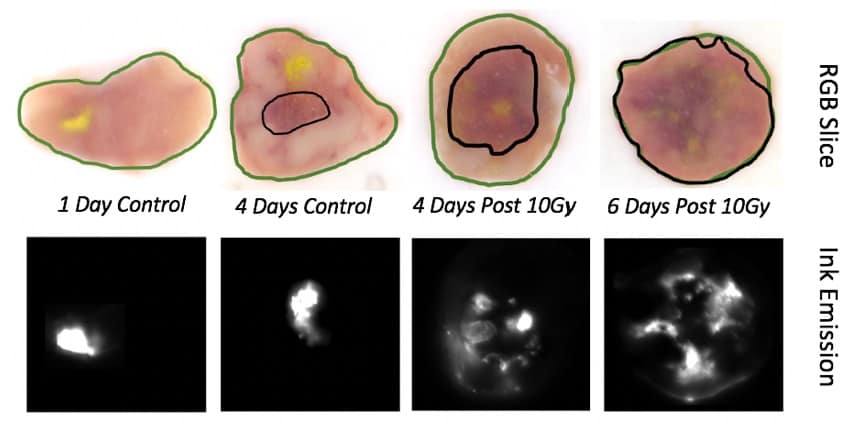
The ex vivo analysis confirmed the trends seen with in vivo CELI. As the volume of necrotic core increased, the fluorescence image slices showed increasing ink diffusion. Analysing the in vivo ink spread revealed a strong correlation with the percentage of necrotic volume, but a weak correlation with total tumour volume (measured manually with callipers).
The researchers conclude that the spread of injected tattoo ink can be related to radiation-induced necrosis, independent of total tumour volume change. They propose that this in vivo imaging system offers potential to track treatment response daily, without interrupting clinical workflow.
Tumour hypoxia tracked in real time during radiotherapy
They note that translating this approach to the clinic will require further developments. These include: increasing in vivo image resolution by moving from widefield irradiation to sheet-scanning illumination; developing a phosphorescent tattoo ink that’s safe for humans; and investigating the effects of injection site to account for inherent tumour heterogeneity.
“Next, we plan to perform more studies of immune infiltration as related to the change in ink diffusion, as well as studies of different types of tumours with variations in stromal density and radiosensitivity,” Soter tells Physics World.