Wherever we look on Earth – even in the most inhospitable places – we find life. But how do organisms manage to survive such difficult conditions? Lorna Dougan explains how physicists are helping to unravel the properties of “extremophile” life
People have long been fascinated by extreme environments and those who explore them. Back in 1969, some 800 million of us tuned in to watch Neil Armstrong set foot on the Moon, while earlier generations thrilled to hear news of Roald Amundsen’s 1911 journey to the South Pole or Edmund Hillary and Tenzing Norgay’s 1953 conquest of Mount Everest. Yet despite the daring of human explorers, it is remarkable that wherever we have ventured on Earth, we have always found that other organisms got there first.
From the inky ocean depths to the driest deserts and the coldest reaches of the polar regions, all of the most inhospitable places on the planet have been colonized. In fact, the greatest explorers of our time may not be humans at all, but the extreme-loving organisms known as “extremophiles” that can survive and thrive in physical and chemical environments that would kill most other forms of life.
The study of extremophiles has a remarkably short history. As recently as 1965, scientific dogma held that no organism could live at temperatures above 55 °C. In that year, however, biologist Thomas Brock began exploring the waters of superheated geothermal pools in Yellowstone National Park, US. Brock observed bacteria growing in springs with temperatures up to 88 °C, and he and his colleagues eventually isolated a previously unknown genus of bacteria, Sulfolobus, that thrives in temperatures of between 75 and 80 °C.
The study of extremophiles really took off after 1977, when researchers using the submersible vessel Alvin discovered an oasis of life more than 2000 m below the surface of the Pacific Ocean off the Galapagos Islands. There is no sunlight at such depths, so instead, the organisms that call this environment home produce organic material via chemosynthesis. In this, they are helped by underwater geysers known as “black smokers”, which spew out superheated water laced with minerals such as sulphide (which gives them their bleak colour) at temperatures that can exceed 400 °C. This water is also highly acidic, with a pH as low as 2.8 – similar to vinegar, which has a pH of 2.2.
Today, the list of known extremophiles is impressively long. As well as the hyperthermophiles and acidophiles mentioned above, there are alkaliphiles, which survive and thrive in habitats with a pH above 9; halophiles, which flourish in highly salty waters such as the Dead Sea; and psychrophiles – cold-loving organisms found in the deep ocean at temperatures of 1–3 °C and at much lower temperatures below (and within) polar ice caps. There are even some organisms that thrive in multiple extreme conditions, such as the bacterium Deinococcus radiodurans. This beautiful example of a “polyextremophile” can survive cold, dehydration, vacuum and acid. Remarkably, it can also withstand a 5000 Gy dose of ionizing radiation with no loss of viability – a staggering amount compared with the 5 Gy dose that would kill a human.
With each discovery, new questions have emerged about the mechanisms that allow these organisms to survive. In our own bodies, extremes of temperature, both high and low, and extremes of pH will destroy DNA and other fundamental biological molecules in our cells. So how do extremophiles manage to tolerate such conditions? The answer is not yet clear, but research at the interface between the physical sciences and biology is providing insights into the complex processes of life in extreme environments. The physics aspect is important because at the cellular and molecular level, biological processes are noisy and stochastic, involving vast numbers of random motions and interactions. Many fields in physics are vital for exploring these complex processes, with knowledge from thermodynamics, statistical mechanics, soft-matter physics and more being brought to bear. These fields provide powerful methods to probe the processes of life in extreme environments in a quantitative and predictive way.
Physics has also given us a different and powerful approach to studying life in extreme environments. The physical properties of extremophiles are often discussed in terms of their equilibrium properties, such as their thermodynamic stability and static structures. We can also gain valuable insights into these systems by examining their kinetic stability, as well as the fundamental forces that govern their functions. We can develop a theoretical description of their behaviour, for example by using scaling laws borrowed from polymer physics. Finally, the development of new instrumentation adapted to extreme pressure, temperature and/or chemical environments has also been integral to advancing our knowledge of extremophile life – particularly since many of these organisms cannot survive in “normal” laboratory conditions.
Keeping it together
In the eyes of biological physicists, globular proteins are colloids, DNA is a stiff polymer and lipids forming cell membranes are surfactants. Extremophiles (and indeed other organisms) are almost entirely made up of “living soft matter”. Physics-based approaches make it possible to measure the forces and fluctuations that shape the dynamics and functionalities of these components, including single biological molecules as well as the larger molecular machinery of the cell.
Proteins, in particular, are fascinating for scientists investigating the extraordinary toughness of extremophile life. These chains of polymers are made up of monomeric units called amino acids, and they provide the workforce that keeps cells functioning – they are, in effect, “bionanomachines”. Proteins fold into a characteristic 3D shape that governs their function, stiffness, elasticity and adhesive properties, all of which play important roles in their various jobs, which include moving other molecules across a cell membrane and providing mechanical robustness in muscle function.
Despite being so useful, though, proteins are rather fragile. To perform their numerous functions, their structures need to be dynamic and flexible. Protein structures are stabilized by a large number of weak, non-covalent interactions, such as hydrogen bonds, ionic interactions, van der Waals forces and hydrophobic interactions. Given that the forces that maintain protein structure are weak, at room temperature the resulting dynamic structure of the protein can be described as malleable, or “mechanically soft” – in other words, they lose their structure when subjected to moderate perturbations. At higher temperatures, molecular vibrations from heat can literally shake a protein apart, unravelling its polymer chain or preventing new proteins from folding correctly once made. Without these protein workhorses, the normal biological machinery of the cell will not operate. How, then, do the proteins in heat-loving extremophiles manage to remain intact?
Applications of extremophiles
Much of the research on extremophiles has focused on answering fundamental questions about how these organisms survive. But there is also a growing industry that seeks to exploit enzymes isolated from organisms that can tolerate extremes of heat, cold, acid, pressure and more. A classic example concerns the heat-loving bacteria species Thermus aquaticus, which was discovered by Thomas Brock and Hudson Freeze in 1969. This bacterium is the source of a heat-resistant enzyme called Taq polymerase, whose job is to assemble a new DNA strand from individual nucleotides.
Taq is now a key component of a technique called polymerase chain reaction (PCR), which can generate thousands (sometimes even millions) of copies of a particular DNA sequence in a few hours using simple equipment. PCR has revolutionized biochemical and genetic research (its creator, Kary Mullis, shared the 1993 Nobel Prize for Chemistry) and it relies completely on the high-temperature adapted capabilities of the Taq enzyme, which must survive cycles of repeated heating and cooling of the DNA between about 68 and 96 °C.
Other applications of extremophiles include using extremophilic enzymes to recover gold, copper and other valuable metals from solid industrial wastes or to degrade toxic compounds. Such enzymes are also being used in agriculture (to modify crops to protect them from cold weather conditions) and to lower costs in the baking industry by making enzymatic reactions more efficient at lower temperatures. Understanding the structure and stability of extremophilic proteins is central to all of these industrial developments.
A myriad of adaptations
One conceptually attractive possibility is that the high thermal stability of proteins in thermophiles might be correlated with the high mechanical rigidity of the protein matrix. But fundamentally all proteins are made from the same collection of amino-acid building blocks. So how can two proteins display such differences in stability and therefore survive different environmental extremes? To answer this question, researchers have drawn on a treasure trove of data known as the protein database (PDB), which contains 3D structural data on proteins.
The first protein structure was solved in the 1950s by John Kendrew and Max Perutz (earning them a shared Nobel Prize for Chemistry in 1962 “for their studies of the structures of globular proteins”), and there are now more than 100,000 biological molecules in the PDB. This information includes common structural motifs, such as alpha-helices and beta-sheets, which had already been predicted by Linus Pauling. Around 90% of the data has been obtained using X-ray crystallography, in which a beam of incident X-rays is diffracted by atoms in the protein. By measuring the intensity and scattering angle of the diffracted beams, crystallographers can obtain a 3D picture of the density of electrons in the protein, and so work out the average position of each atom in the crystal. Most of the remaining 10% of structures in the PDB have been resolved using nuclear magnetic resonance spectroscopy, while a few structures have been solved by firing a beam of electrons through a cryogenically cooled sample, to yield a high-resolution image.

By comparing protein structures in the PDB, researchers have uncovered a myriad of adaptations that keep proteins stable and active under extreme conditions. For example, Michael Danson, a structural biologist at the University of Bath’s Centre for Extremophile Research, and co-workers have shown that heat-adapted (thermophilic) proteins tend to have a more tightly packed hydrophobic core and greater electrostatic interactions than their middling-temperature (mesophilic) counterparts. Cold-adapted (psychrophilic) proteins, in contrast, have a reduced hydrophobic core and a less charged protein surface, which may help them to stay flexible and active at low temperatures. Meanwhile, detailed studies by Oscar Millet and co-workers at the CIC bioGUNE lab, Spain, on salt-adapted (halophilic) proteins have shown that structures featuring amino acids with short “side chains” are preferred, as they reduce the interaction surface between the protein and the solvent. This is thought to be an advantage in an environment where water molecules are in short supply because so many of them are bonded to salt ions.
Among the other types of extremophiles, the pressure-adapted piezophiles are of particular interest to physicists. One of the first to study how proteins fall apart, or denature, at high pressure was Percy Bridgman, who won the 1946 Nobel Prize for Physics “for the invention of an apparatus to produce extremely high pressures and for the discoveries he made therewith in the field of high-pressure physics”. After subjecting an egg to a pressure of 7000 atmospheres, Bridgman found that the egg appeared as though it had been hard boiled in water – a discovery that fuelled interest in using high pressures to preserve food.
More recently, experimental studies have begun to probe the high-pressure limits on bacterial survival. For example, Paul McMillan and co-workers at University College London have found that the bacterium Shewanella oneidensis MR-1 can survive pressures into the gigapascal range – more than 10,000 times higher than atmospheric pressure at sea level. The survival mechanisms for such bacteria are not well understood, but new technologies may help. Nick Brooks and co-workers at Imperial College London, for example, are developing instruments to study the effect of high pressure on the structure and dynamics of biological systems. Their current projects include a device that makes it possible to carry out X-ray diffraction experiments at very high pressure, along with a platform for obtaining dynamic information under high pressure.
Folding into place
While we now know a lot about how the structures of proteins in extremophile organisms have adapted, we have less information (particularly quantitative data) on the conformational dynamics and flexibility of these proteins. An important area of study is what happens when proteins fold. Protein folding is a stochastic process by which a polypeptide chain transforms itself from a random coil into a characteristic and functional 3D structure.
Studying how proteins fold in extreme environmental conditions presents physicists with a wonderful opportunity to explore the balance of entropic and enthalpic components that govern protein stability
We are not yet able to predict the correct folded structure of a protein, or how it gets there, simply by looking at its sequence of amino acids. However, we do know that folding involves a delicate interplay between global geometry and local structure, and that it is induced by hydrophobic interactions between non-polar regions of the protein chain (as well as by other mechanisms such as hydrogen bonding). We also know that when a protein folds, it loses a lot of the entropy associated with the numerous possible conformations it could have. This loss of conformational entropy is balanced by the increase in the enthalpy that results when new bonds form, including hydrogen bonds and ionic interactions. Studying how proteins fold in extreme environmental conditions thus presents physicists with a wonderful opportunity to explore the balance of entropic and enthalpic components that govern protein stability.
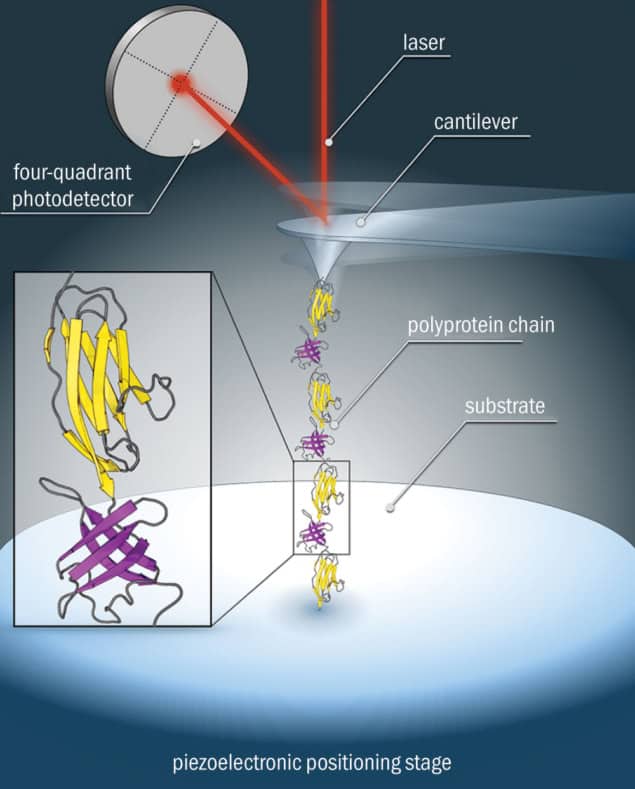
The advent of single-molecule force spectroscopy (SMFS) in the mid-1990s has helped to reveal the molecular mechanisms of protein folding. One common SMFS technique uses an atomic force microscope, with one end of the protein tethered to a gold surface, while the other end is attached to the tip of a small, flexible cantilever. As the tip is moved away, the protein stretches out and unfolds, and the cantilever bends. By measuring the cantilever’s deflection with a laser and photodetector, the force needed to unfold the protein can be calculated, and the size of this force reveals the protein’s mechanical stability. Force measurements can also be used with theoretical models to determine other properties of the protein, such as its stiffness.
SMFS experiments have provided remarkable insight into the mechanical stability of proteins, revealing details about the relationship between protein structure and stability, as well as directly measuring protein unfolding and folding kinetics. Thanks to this work, we can now probe the importance of non-covalent interactions to the mechanical and thermodynamic stability of extremophilic proteins, including in conditions that mimic those in which the extremophile organisms lived (high and low temperature, and solution pH).
At the University of Leeds, we have been using SMFS to manipulate a protein called TmCsp (commonly known as the “cold-shock protein”) that is found in a hyperthermophilic bacterium, Thermotoga maritima (figure 1). In measuring the forces required to unfold TmCsp at different temperatures, we have seen the protein’s spring constant drop as the temperature rises, reflecting the enhanced malleability of the structure. These findings actually run counter to the hypothesis that proteins from thermophiles should have rigid structures resulting from improved packing and increased numbers of ionic interactions. Instead, our experiments suggest that malleability may be an essential design feature of heat-adapted proteins – perhaps by giving the protein a way of recovering its structure under extreme high-temperature conditions. Further studies will seek to uncover more details of the “energy landscape” of folding in proteins from extremophilic organisms, and to understand the importance of intermediate stages in the folding process.
New frontiers for life
Outside the biological-physics lab, the hunt for new extremophiles continues. The exploration of Antarctica, for example, has revealed previously unknown areas of groundwater and subsurface liquid water, some of which may house new examples of extreme life. Specialist facilities such as the UK’s Boulby International Subsurface Astrobiology Lab – the world’s first permanent deep subsurface laboratory – also provide opportunities to complete experiments in extreme conditions. Each discovery gives us a clearer understanding of the conditions required for life and extends the limits of what we know is possible. Extremophile discoveries are also helping to fuel the search for life beyond our own planet. By advancing our understanding of how molecules and organisms respond to extreme environments, we also learn more about the potential habitability of other worlds – including Mars, which according to recent evidence may be home to flowing water.

Back here on Earth, studies of extremophiles and extremophile proteins may prove important for the development of new biomaterials. Designing materials that are thermodynamically and mechanically robust, yet are also malleable or rigid enough to suit particular applications, is a big challenge, and to fully exploit proteins as self-assembling components in such new materials, we will need to expand the toolbox of available proteins. Proteins from extremophilic organisms therefore present interesting opportunities for researchers seeking to engineer robust synthetic biological materials.
While the bacteria and their single-celled cousins, the archaea, are the true masters of extremes, as the field of synthetic biology continues to grow, we may eventually need to add a new domain to the tree of life. In creating such a domain – which has already been given a suggested name, synthetica – we can take inspiration from the many extremophiles that have been found in the bacteria and archaea domains, and perhaps some from our own domain, eukarya. Advanced techniques such as genome sequencing and super-resolution microscopy, as well as exploration of the macromolecular organization of the cellular machinery and collective motion, will undoubtedly shed light on the mystery of how extremophiles survive and adapt to some of the toughest conditions on the planet.