Recent developments in 3D printing of metallic biomaterials enable fabrication of orthopaedic implants tailored for bone reconstruction following trauma or bone tumours, or for use in joint replacements, spinal implants and reconstructive surgery. It’s also possible to fabricate these implants with surface properties that can promote bone-tissue regeneration and minimize the risk of implant-associated infections.
Insertion of any implant in the human body, however, triggers an immune response that can influence the above-mentioned biofunctionalities.
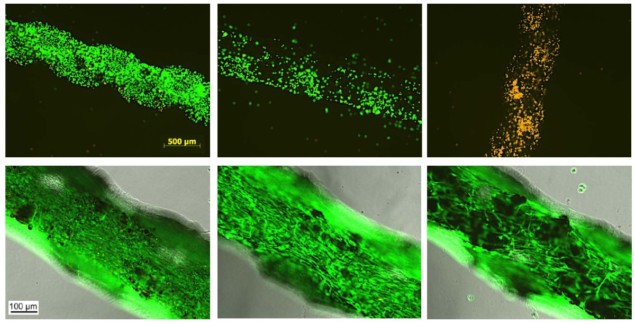
Aiming to minimize the risk of implant-associated infections, a multidisciplinary research team from the Netherlands involving engineers from TU Delft and biologists from Erasmus MC has examined the in vitro immune response triggered by porous titanium implants printed using selective laser melting (SLM). The team examined three types of SLM implants: untreated; surface biofunctionalized using plasma electrolytic oxidation (PEO); and PEO-biofunctionalized in the presence of silver nanoparticles (PEO+Ag) to provide antibacterial functionality (Biomed. Mater. 10.1088/1748-605X/ab7763).
“Most of the research on implants is focused on how they can stimulate formation of the new tissue, with very scarce data on the inflammatory response they elicit,” explains Lidy Fratila-Apachitei from Delft University of Technology. “Our implants are designed to stimulate bone regeneration and prevent implant associated infections. The inflammatory responses triggered by these implants may affect both these biofunctionalities. Therefore, we were interested to know how inflammatory cells (macrophages) respond to our implants.”
In vitro behaviour
Implanted biomaterials trigger responses that activate both pro-inflammatory and anti-inflammatory, pro-healing macrophages. The fine balance between these two types is influenced by the biomaterial’s properties. To investigate this, the researchers examined the response of human peripheral blood monocyte-derived macrophages cultured on the three different titanium implants.
The untreated implants triggered a strong pro-inflammatory response in macrophages, including relatively high levels of genes that may have detrimental effects on healing, combined with early anti-inflammatory effects. PEO-treated implants showed a higher potential to induce pro-healing macrophages, while incorporation of silver nanoparticles led to cytotoxic effects.
Cell type matters
The team also examined the culture of human mesenchymal stromal cells on the same implants and observed that they survived on all surfaces. This finding indicates that the PEO+Ag implants were not cytotoxic for these cells, in agreement with previous research, and suggests different levels of silver toxicity for different cell types.
The researchers emphasize that macrophage sensitivity to the presence of silver nanoparticles may lead to a compromised immune response in vivo. Therefore, the team is working on finding the optimum concentration of silver nanoparticles that would ensure both macrophage viability and antibacterial function of the implant. Thereafter, the researchers will continue to explore the immunomodulatory effects observed with the aim of achieving immune responses that may favour bone regeneration.



