There is a long tradition of physicists and physics-based techniques making important contributions to biology and medicine. Here the director of the National Institutes of Health, one of the world’s foremost biomedical research centres, argues that this tradition must go on.

The aim of most biomedical research is to uncover new knowledge that will lead to better health. At the National Institutes of Health (NIH) in the US we do this by supporting research on the prevention, detection, diagnosis and treatment of disease and disability, from the rarest genetic disorder to the common cold, as well as research on the basic principles of biology.
In this article I would like to discuss my conviction that we can only wage an effective war on disease if the scientific community harnesses the energies of many disciplines, not just biology and medicine. These allied disciplines range from mathematics, engineering and computer science to sociology, anthropology and the behavioural sciences. But the weight of historical evidence and the prospects for the future place physics and chemistry most prominent among these disciplines.
Physics and biology
I will discuss the effects of physics on the medical sciences from three perspectives. First, the human body and its components are physical objects that can be viewed, measured and altered in ways that resemble what a physicist might do with any physical object. Second, I will remind you of an enormously important phase in the history of biology in which physicists transformed the study of living things by helping to discover the principles of heredity. Third, I will describe some contemporary problems in the biomedical sciences that I believe present challenges to physicists, young and old. I will also explain the ways in which the NIH is attempting to ease the path from a formal training in physics to an active, investigative role in biomedical sciences.
I am only the latest in a long line of commentators who have made the really quite obvious point that, for at least several hundred years, physicists – and especially their principles, methods and machines – have been illuminating our views of the human body and of every other living thing.
This notion was brought home to me very early in life when my father – a general practitioner whose office was directly connected to our house – showed me how X-rays and fluorography could reveal the bones and lungs of our pets and his patients, and help make diagnoses of disease. Röntgen and Edison had been pioneers in this respect. The significance of using the discoveries of physics to perceive biological function was further impressed on me at college, when one of my first independent projects required that I try to explain the repeating peaks and valleys of my electrocardiogram as a record of voltage changes in the salty sea of the human body. And at medical school I learned that the doyens of our biochemistry department had become famous by being the first to tag red blood cells with easily detected radioisotopes to learn how long such cells survived in the body.
These few personal memories are just a sampling of the hundreds of physics-based methods that have been applied to view living bodies without the disruption of anatomical dissection or to visualize very small components of living things.
A more systematic rendering of this topic was offered by the distinguished Stanford physicist Robert Hofstadter, in a talk to the National Academy of Sciences in 1983 (see table). It is instructive to note how many of the methods can be classified as techniques that permit us to visualize the inside of the human body at successively higher levels of resolution, or allow us to see smaller and smaller elements of bodily components.
The methods of “macro-imaging” include conventional X-radiology, computerized tomography scanning, ultrasound, positron-emission tomography (PET) and magnetic resonance imaging (MRI). The impact of these procedures on medical practice is unquestioned and continues to grow as new methods and new applications appear. Two recent examples convey the exciting potential for both clinical and investigative work – the combined use of PET and MRI to provide images of the human brain at work (figure 1), and the use of MRI to analyse both structural and functional characteristics of the human heart in diseased states.
“Micro-imaging” began with the use of optical principles to devise the light microscope, but has progressed to much higher levels of resolution with electron microscopy, X-ray crystallography and nuclear magnetic resonance.
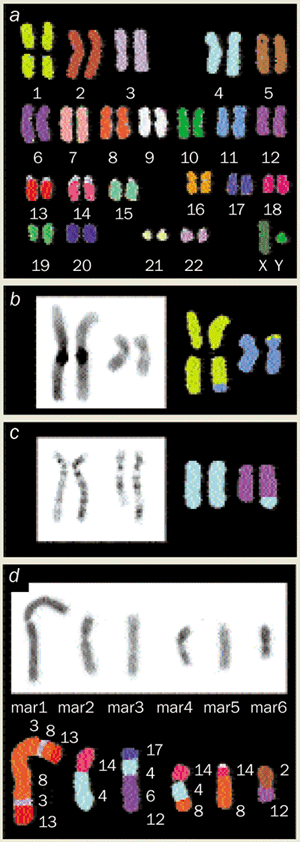
Sometimes a collection of methods proves important, as in the combined use of molecular hybridization, fluorochrome chemistry, wave optics, and computer science in “spectral karyotyping”. This procedure allows the rapid identification of each of the 23 pairs of normal human chromosomes and also the origins of recombined chromosomes that often appear in cancer cells (figure 2).
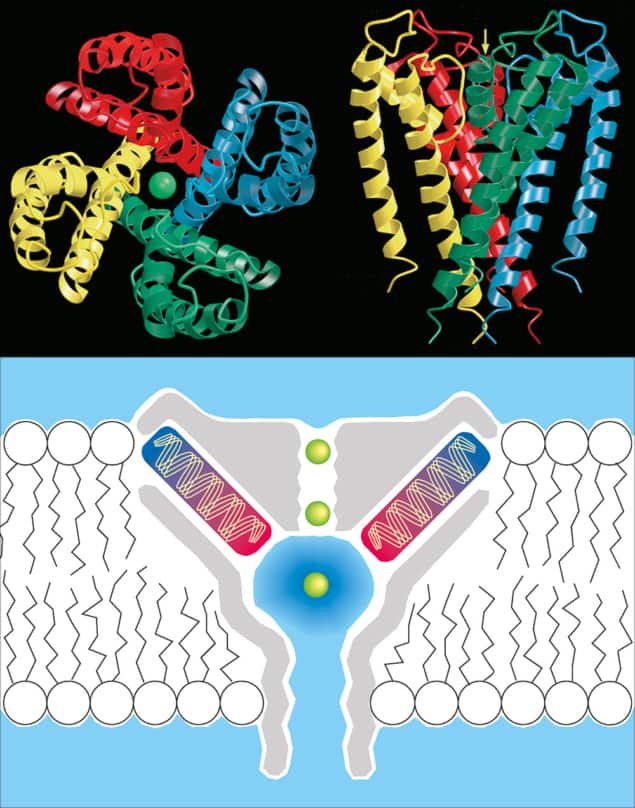
Long-awaited success in using a time-honoured technique, X-ray crystallography, to resolve the structure of proteins embedded in biological membranes has recently transformed the study of cell function and disease. I used an important example of this progress – the analysis by Rod MacKinnon and co-workers at Rockefeller University in New York (see Doyle et al. in further reading) of potassium channel proteins to understand how the channels can be so efficient and yet so selective (figure 3) – when justifying further investments in research to Congress this year.
Despite the centrality of such contributions of physics to modern biology and medicine, I recognize the danger that my emphasis might be interpreted as limited and perhaps even insulting, because (some might say) I have portrayed physicists as merely the developers of tools of measurement that allow biomedical scientists to do the really important work. There are reasons for my sensitivity to this issue: in a 1967 commentary on the role of physics in biology and medicine, for example, Sergei Feitelberg, a physicist from Mount Sinai Hospital in New York, noted that while such “spectacular developments created a clear and unequivocal need for physicists and their help, the role of the physicist was that of a glorified technician engaged in methodology and instrumentation, dignified only by the strangeness of his doings and the mysteriousness of his tools”.
I do not accept that interpretation. In fact, I would argue that we need to show our appreciation of physics-based technology by investing NIH funds more aggressively in its development. We have begun to do just that through a new Bioengineering Consortium and a trans-NIH emphasis on technology development. Still, I would like to address a deeper set of contributions that physics makes to biology – through the efforts of physicists who themselves seek to understand the rules of living systems.
Correlations between physics and medicine
| Physics | Medicine |
| Statics (mechanics) | Orthopaedics |
| Dynamics (mechanics) | Heart motion |
| Elasticity and strength of materials | Orthopaedics |
| Fluid statics | Blood pressure |
| Fluid dynamics | Blood flow in vascular system |
| Surface tension | Capillary action |
| Sound and acoustics | Stethoscope, ultrasound, acoustic microscope |
| Electricity | All life processes, ion transfer at membranes |
| Magnetism | Nuclear magnetic resonance imaging |
| Light and optics | Light microscopy, laser therapy, fibre optics |
| Heat and thermodynamics | Heat balance |
| Kinetic theory and statistical mechanics | Brownian motion, osmosis, diffusion of gases |
| Atomic physics and spectroscopy | “Chemical shift” in NMR imaging, lasers in medicine |
| Molecular physics | Genetics, antibodies, protein structure, electron microscope |
| Ultraviolet and infrared energy | Skin treatment and imaging |
| X-rays | Radiology, CT imaging |
| Quantum mechanics | Electron diffraction microscope |
| Relativity | Synchrotron radiation imaging |
| Crystallography | Structure of proteins |
| Solid-state physics and semiconductors | Computers in medicine, scintigraphy |
| Nuclear physics | Radioisotope labelling, nuclear medicine, radiation therapy |
| Radioactivity | Positron emission tomography (PET) |
| Elementary particle physics | Pion therapy |
| Accelerators, cyclotrons, etc | Tumour therapy, Hodgkin’s disease |
| Astronomy and astrophysics | Discovery of helium, treatment of asthma (obsolete) |
This table was presented by Robert Hofstadter of Stanford University at a conference on biological imaging organized by the National Academy of Sciences in October 1983. Hofstadter had shared the Nobel Prize for Physics in 1961 for his work in nuclear physics. Many new physics-based techniques have become important in biology since then, for example various image capture and analysis techniques developed by astronomers and astrophysicists.
Physicists, heredity and the rise of molecular biology
Exactly 50 years ago, in a speech entitled “A physicist looks at biology”, Max Delbruck, a leading physicist who had made a conversion to biology some years earlier, attempted to describe the transition. In the speech, delivered to the 1000th meeting of the Connecticut Academy of Arts and Sciences, Delbruck said: “A mature physicist, acquainting himself for the first time with the problems of biology, is puzzled by the circumstance that there are no ‘absolute phenomena’….The animal or plant or micro-organism he is working with is but a link in an evolutionary chain of changing forms, none of which has any permanent validity. Even the molecular species and the chemical reactions which he encounters are the fashions of today to be replaced by others as evolution goes on. The organism he is working with is not a particular expression of an ideal organism, but one thread in the infinite web of all living forms, all interrelated and all interdependent. The physicist has been reared in a different atmosphere. The materials and phenomena he works with are the same here and now as they were at all times and as they are on the most distant star.”
Delbruck had been a student of Niels Bohr and then a powerful proselytizer for biology. With the assistance of Bohr’s book Light and Life and, more importantly, Schrödinger’s book What is Life?, he attracted many other physicists to biology. The effects of his missionary zeal were powerful – not just because some very smart people started to do biology, but because they brought to biological problems a quantitative, analytic approach – an approach that created the atmosphere in which principles of molecular biology were discovered by seeking the physical basis of heredity.
The leading physicist Leo Szilard was among the converts, and claimed that what physicists brought to biology was “not any skills acquired in physics, but rather an attitude: the conviction which few biologists had at that time, that mysteries can be solved” (see Fleming in further reading).
Delbruck and his friends were gripped by some fundamental questions: what is the physical form in which hereditary information is stored? How is it reproduced when a cell divides, or when a single virus particle invades a cell and makes hundreds or thousands of copies of itself? How is the information reassorted during sexual reproduction? How does the information change when mutations occur?
Answers to many of these questions came from the “phage school” that Delbruck founded. The phage school was a group of former physicists and some biologists who shared his passion for reducing the problem of heredity to simple rules, physical entities and conserved energy by studying the replication and genetic behaviour of bacterial viruses (also called bacteriophage or “phage”) in their bacterial hosts. The studies culminated in findings that form the pillars of modern molecular biology: the identification of deoxyribonucleic acid (DNA) as genetic material, a description of the physical organization of DNA through X-ray crystallography, the deduction of the principles of base pairing and the strategy of replication from the organization of the double helix, and the deciphering of the genetic code as triplets chosen from a set of four nucleotides.
Delbruck and his phage school were important, but there were, in fact, multiple intellectual lineages connected with physics that helped to create the modern world of molecular biology (see Keller in further reading). For instance, Warren Weaver was a mathematical physicist turned science administrator who, in 1932, first used the term “molecular biology”. He chose this phrase because he foresaw “that the moment would arrive when the distinction between chemistry and physics and even mathematics on the one hand and biology on the other would be so illusory and in fact so unfortunate” that he did not want to use the word “biology” to describe the programmes he was supporting at the Rockefeller Foundation.
British scientists with a strong physical bent, such as Astbury, Bragg and others, used X-ray diffraction to study the organization of fibres of many kinds, mainly proteins found in textiles, in an intellectual lineage that led to Wilkins and Franklin and, of course, DNA. The American geneticists T H Morgan and H J Muller used physical agents – namely X-rays – to induce mutations in fruit flies. Muller’s affinity for the principles of physics was especially strong. He was fond of noting the potential similarities of mutation of genes to transmutation of elements, calling the prospect of understanding these events in physical terms “the two keystones of our rainbow bridges to power” (see Carlson in further reading)
Bringing physics, not just physicists, to biology
To the birth of modern molecular genetics, physicists contributed their analytic skills but they were not really doing physics, and many were not even using the computational or imaging tools of physics as many biologists do. Delbruck and his colleague Salvador Luria laboriously counted virus infections by hand and eye, just like any other biologist. But contemporary biology, especially the deciphering of genomes by nucleotide sequencing, is about to change that. Biology is rapidly becoming a science that demands more intense mathematical and physical analysis than biologists have been accustomed to, and such analysis will be required to understand the workings of cells.
This change was clearly foreshadowed in Delbruck’s 1949 lecture in Connecticut. He first described his awe at the complexity of biology: “The closer one looks at [the] performances of matter in living organisms the more impressive the show becomes. The meanest living cell becomes a magic puzzle box full of elaborate and changing molecules, and far outstrips all chemical laboratories of man in the skill of organic synthesis….”
But Delbruck also sounded a warning: “Biology is a very interesting field…[because of] the vastness of its structure and the extraordinary variety of strange facts…but to the physicist it is also a depressing subject, because…the analysis seems to have stalled around in a semi-descriptive manner without noticeably progressing towards a radical physical explanation…we are not yet at the point where we are presented with clear paradoxes and this will not happen until the analysis of the behaviour of living cells has been carried into far greater detail.”
In the past 50 years, and especially in the past 20, molecular and cell biologists have moved much closer to the “radical physical explanation” of cell behaviour that Delbruck sought. Certainly the chemical elements – especially the genes, the ribonucleic acid (RNA), and the proteins – and some of their basic functions are coming into view. What is lacking is a sense of how these functions are integrated to allow cells to manifest their physiological traits.
I would like to mention three of the several arenas of biology in which I believe the skills of physicists and their close cousins can be most productively used.
The first is perhaps the most reductionist. Methods are now available for examining the physical and chemical properties of single macromolecules and single complexes of large molecules. These advances are important because they avoid the need to synchronize a population of molecules to measure function. Several of these methods and their applications are reviewed in a special section on “single molecules” in the 12 March 1999 issue of Science. They include laser traps (“optical tweezers”) to study the energetics of molecular motors used for transport, for contraction and for flagellar motion. Steven Chu of Stanford University, who shared the 1997 Nobel Prize for Physics, has made significant contributions to this problem in collaboration with his colleague the cell biologist Jim Spudich.
Laser traps can also be used to measure the force of an enzyme complex, such as the one that copies DNA sequences into RNA. Fluorescence spectroscopy and scanning tunnel microscopy can visualize the conformation of single large molecules, and methods now in development may soon be able to determine the order of bases in single, long DNA molecules.
Second, the computational experience of physical scientists is needed to help interpret complex data sets and the process of “gene expression”. One of the consequences of projects to sequence the genomes of human beings and many other species is the opportunity to understand the processes by which the genes of an organism are expressed. Such information can help us to understand, for example, why some cells develop into muscle tissue, while others become brain cells. New methods, built on the availability of a piece of DNA from each gene, allow us to measure the extent to which genes are read to form RNA (and subsequently protein) in different tissues and under different environmental conditions.
These micromethods, called “expression arrays”, are coming into wide use to study bacteria (with several hundred to a few thousand genes), yeast (with about 6200 genes), worms (with about 19 100 genes) and vertebrates (which are predicted to contain about 80 000 genes). Some progress has been made through computer-based “cluster analysis” (see Eisen et al. in further reading) to begin to interpret the voluminous data that such experiments generate, but biologists are generally unused to such complex data sets. Recently, I spent an evening at the Carnegie Institution’s observatory at La Serena in Chile, watching astrophysicists gather amazingly similar data sets to search for supernovae and to measure the chemical composition of distant stars. We are all likely to benefit from an interdisciplinary exchange of computational approaches.
The third area of opportunity for physicists in biology is the one that most closely approaches Delbruck’s goal of developing a “radical physical explanation” for cell function. In the past 20 years, mainly through efforts to identify the genes and proteins that control cell growth and responses to hormones, biomedical investigators have constructed many so-called signalling pathways that link molecular interactions at the cell surface to changes in gene expression in the nucleus.
While there is consensus that these linear pathways are over-simplified, the way forward is far from clear. The pathways doubtless have many unrecognized components; the information is certainly flowing between, not just along, the several pathways; and the pathways are probably regulated in complicated ways through feedback mechanisms and other means. A few investigators are beginning to grapple with these issues (see Bhalla and Iyengar, and Weng et al. in further reading) but there is an obvious need to apply experiences with potentially analogous complex machines.
Finale: moving between disciplines
In talking about the effects of one field on others, I have generally ignored the “boundary problem” – how do we distinguish among fields? We do this now, in part, by self-identification, just as we deal with ambiguity about race, ethnicity and religion. Self-identification in science is commonly linked to the source of one’s graduate degree, and departmental names on diplomas can become limits to exploration in adjacent fields. But many of us in biology expect that, as studies of cells and molecules become more obviously in need of several disciplinary approaches, it will become increasingly difficult to label the science and to predict the kinds of degrees the people doing it should have.
At the NIH, we have become concerned about how people should be trained in college and in graduate studies to pursue biological problems over the next 50 years, and we are discussing the need to study this issue with the National Research Council. I also agree with Leon Lederman, who has been leading the movement to establish a more logical order for teaching the sciences in US high schools: that is physics, chemistry and then biology. But these activities will come to fruition only after many years, and it is important to also consider the more immediate need to transport intellects across artificial disciplinary boundaries.
I sense increasing interest in attempting to open borders that have been traditionally hard to cross. In the US, workshops on computational biology and approaches to complex systems have recently been organized by the National Institute of General Medical Sciences and the Department of Energy. New funding opportunities for interdisciplinary work are available through the Bioengineering Consortium and other programmes at the NIH. At present, total NIH funding of physics projects is estimated to be about $287m.
There are many anecdotal accounts of successful interdisciplinary training programmes. Within our intramural research program at the NIH, physicists and physics trainees from the US and abroad do graduate thesis work, take courses in biological topics, and engage in post-doctoral training that promotes interactions with biologists and clinicians. Much of this activity occurs under the direction of some of our most prestigious scientists – such as Ad Bax, Bob Balaban, Bill Eaton and Adrian Parsegian – and includes work on small-molecule and protein NMR, brain and cardiac MRI, and other topics, leading to good job prospects for trainees.
On the occasion of the 100th anniversary of the American Physical Society, I thank physicists for their many contributions to biology and medicine – for providing the tools that allow us to see and probe living things, and for training great minds that have uncovered some of the most fundamental principles of biology. I now encourage physicists to work collaboratively with biologists as we strive to achieve Delbruck’s “radical physical explanation” for biological systems.



